Nanofiber-hydrogel composites for enhanced soft tissue replacement and regeneration
a technology of nanofiber and hydrogel, applied in the field of composite materials, can solve the problems of donor site defects, fibrosis and encapsulation, and difficult treatment of soft tissue defects resulting from trauma, oncologic resection, or congenital malformations by conventional means, and achieve the effect of improving the quality of soft tissue reconstruction and improving the properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
orming Composite with Reduced Inflammation Profiles
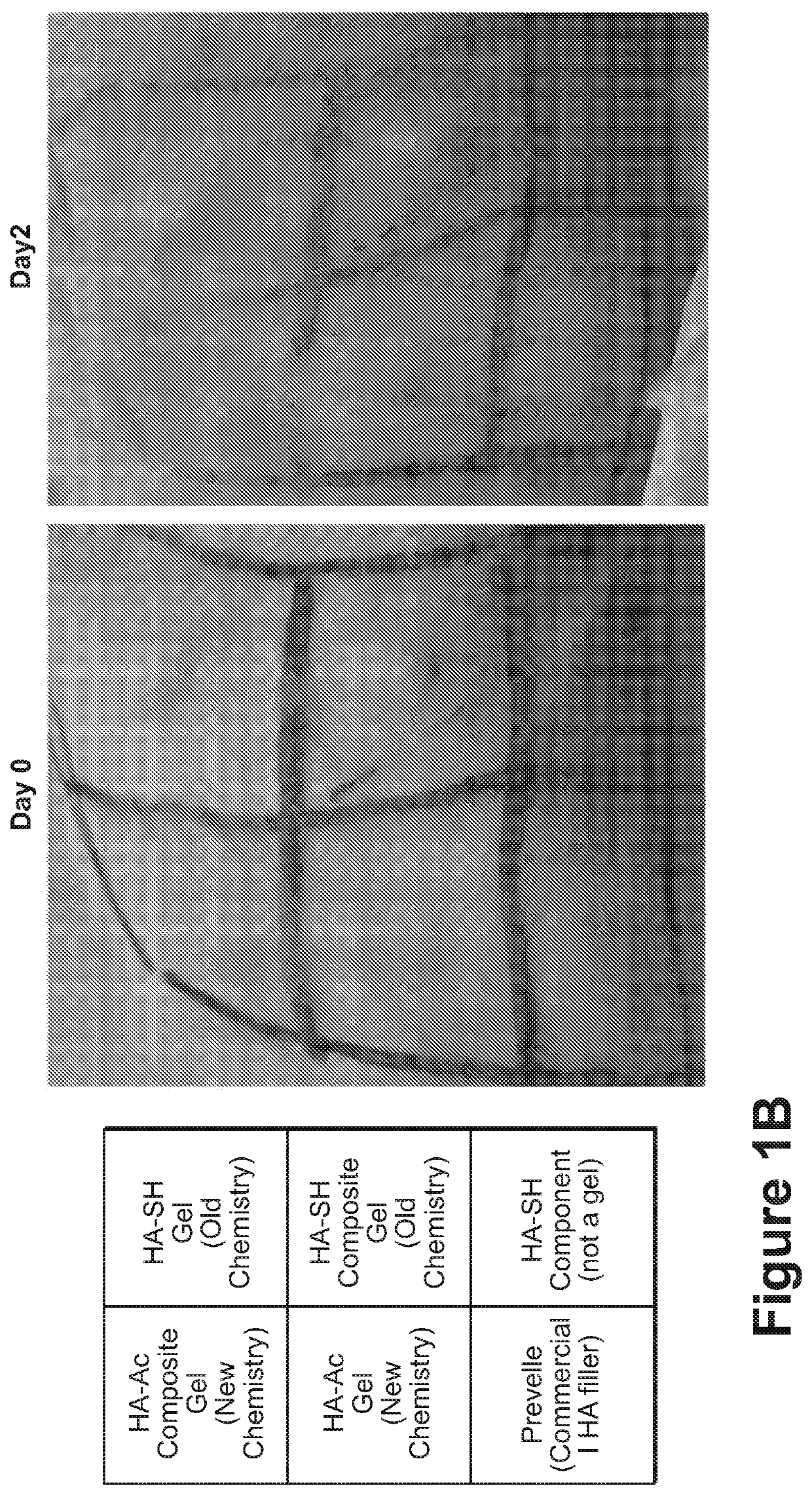
[0243]An in situ-forming composite was developed comprising 5 mg / mL of thiolated HA (HA-SH), 10 mg / mL of polycaprolactone (PCL) fibers and the concentration of PEGDA set to match the thiol concentration 1:1 with the combined acrylate and maleimide concentrations (5 mg / mL). The components were mixed together to react approximately 30 minutes before surgery in order to begin gelation, with the bulk of the gelation being completed in situ.
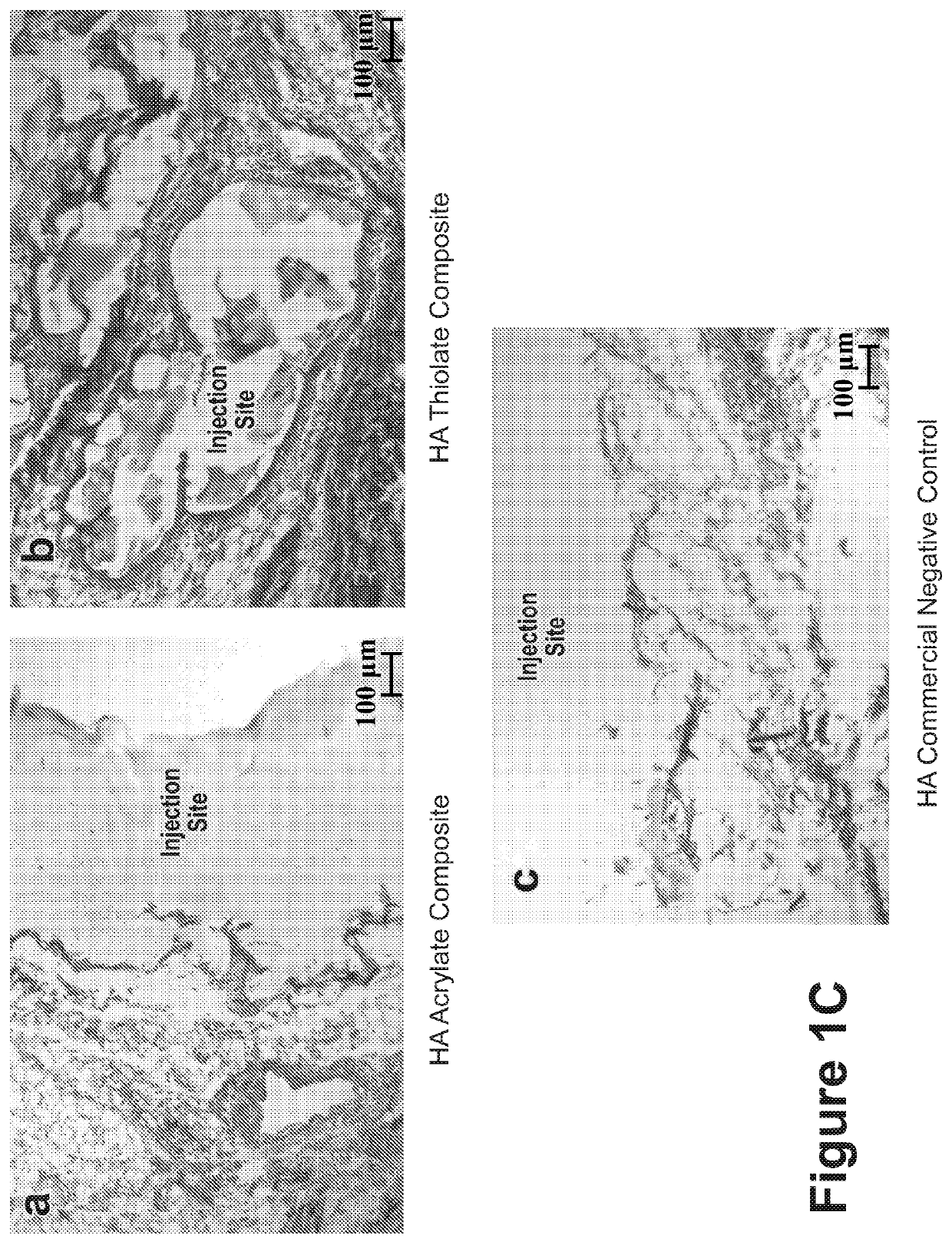
[0244]While gelation success was achieved in vitro and in animals (rodents and rabbits by subcutaneous (s.c.) injection), the chemistry preparation utilizing the thiolated-HA caused short-term moderate inflammation when injected in the subcutaneous rabbit model. In order to produce a composite formulation with reduced inflammation, the reactive groups between the HA and PEG were reversed, keeping an earlier formulation comprising a fiber-maleimide component.
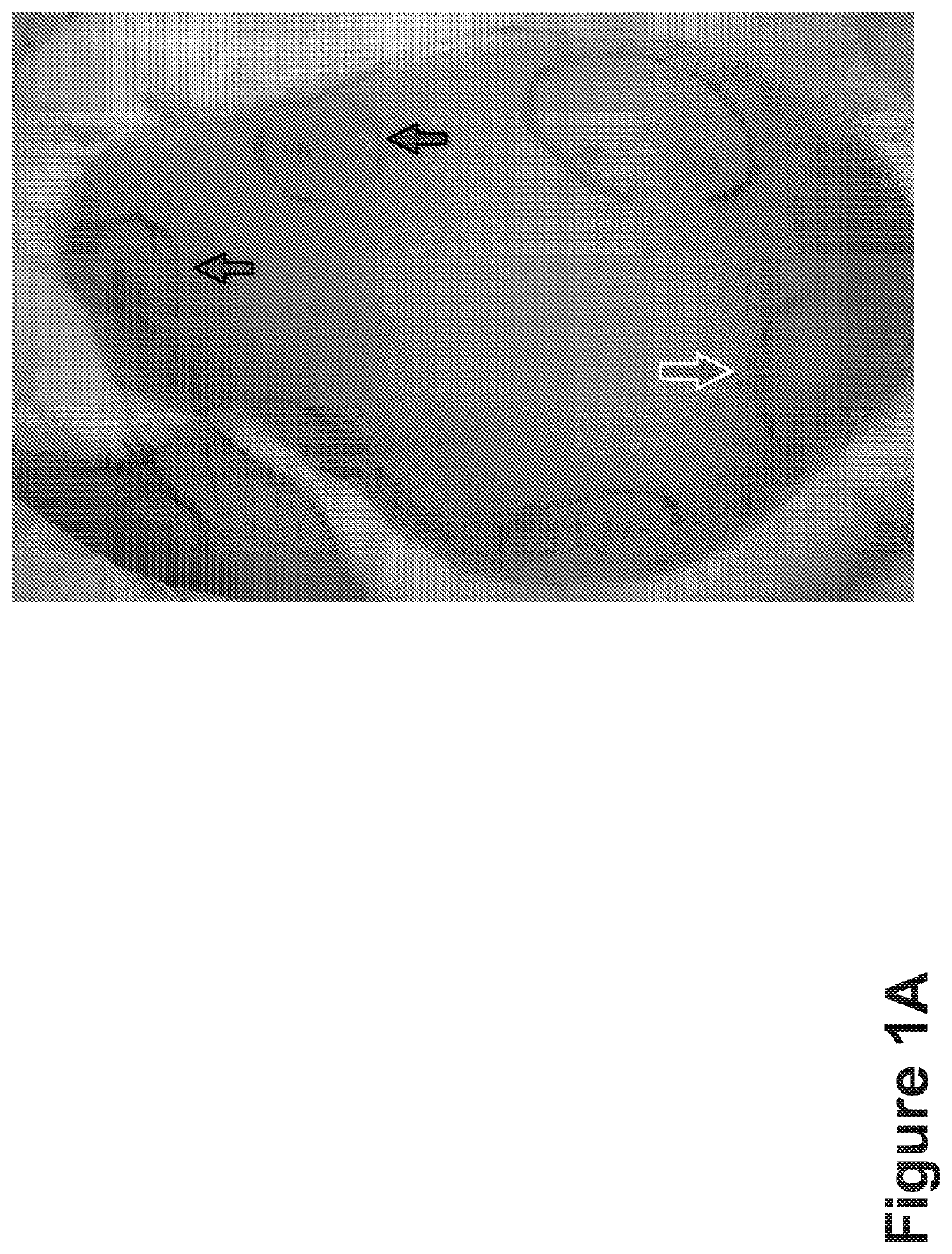
[0245]As shown in FIG. 1A, the upward arrows...
example 2
ed Composite Beads with New Composition
[0252]To improve storage stability and make the gel simpler and more consistent for the end-user, a gel was formed comprising a pre-reacted, beaded formulation, wherein the formulation (7 mg / mL HA-Ac, 8 to 10 mg / mL of fibers with maleimide, and 6.9 m / mL of PEGSH) is fully reacted in bulk at 37° C. By pre-reacting the gel during manufacturing, the labile functional groups did not need to be protected, and the need for extensive mixing and curing by the end-user was removed.
[0253]The bulk gel is formed into 150 or 250 μm beads, then lyophilized in an isotonic solution of sucrose, trehalose, and sodium chloride (to protect the microstructure during the drying process and extend the product's shelf life). The gel beads are then reconstituted with water and within seconds are ready for injection, with the same storage modulus as prior to lyophilization. Optical microscopy images of the beaded composite are shown in FIG. 2. FIG. 2A shows the composit...
example 3
emical Characterization of Composite Beads
Determination of Size Distribution
[0255]Diameters of composite beads were measured along the longest axis of the particles under a confocal microscope image. The analysis were performed counting 51 particles. Histogram of the particles (FIG. 2G) gives the average bead size as 209.41±62.27 μm. FIG. 2H demonstrates confocal microscope images of beads of size ˜75 μm, ˜150 μm and ˜200 μm by measuring the longest axis within the particle.
[0256]Further characterized bead size distribution is performed using image analysis program that will use edge detection for more consistent measurements.
[0257]In some embodiments, different mesh size sieves used to process the bulk composite can yield to different histograms for the bead sizes.
[0258]In an alternative embodiment, SEM (Scanning Electron Microscopy) is used to image composite beads. Staining of the hydrogel or fibers may be required during the imaging process.
Assessing Injectability Based on Size ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| mean length | aaaaa | aaaaa |
| mean size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com