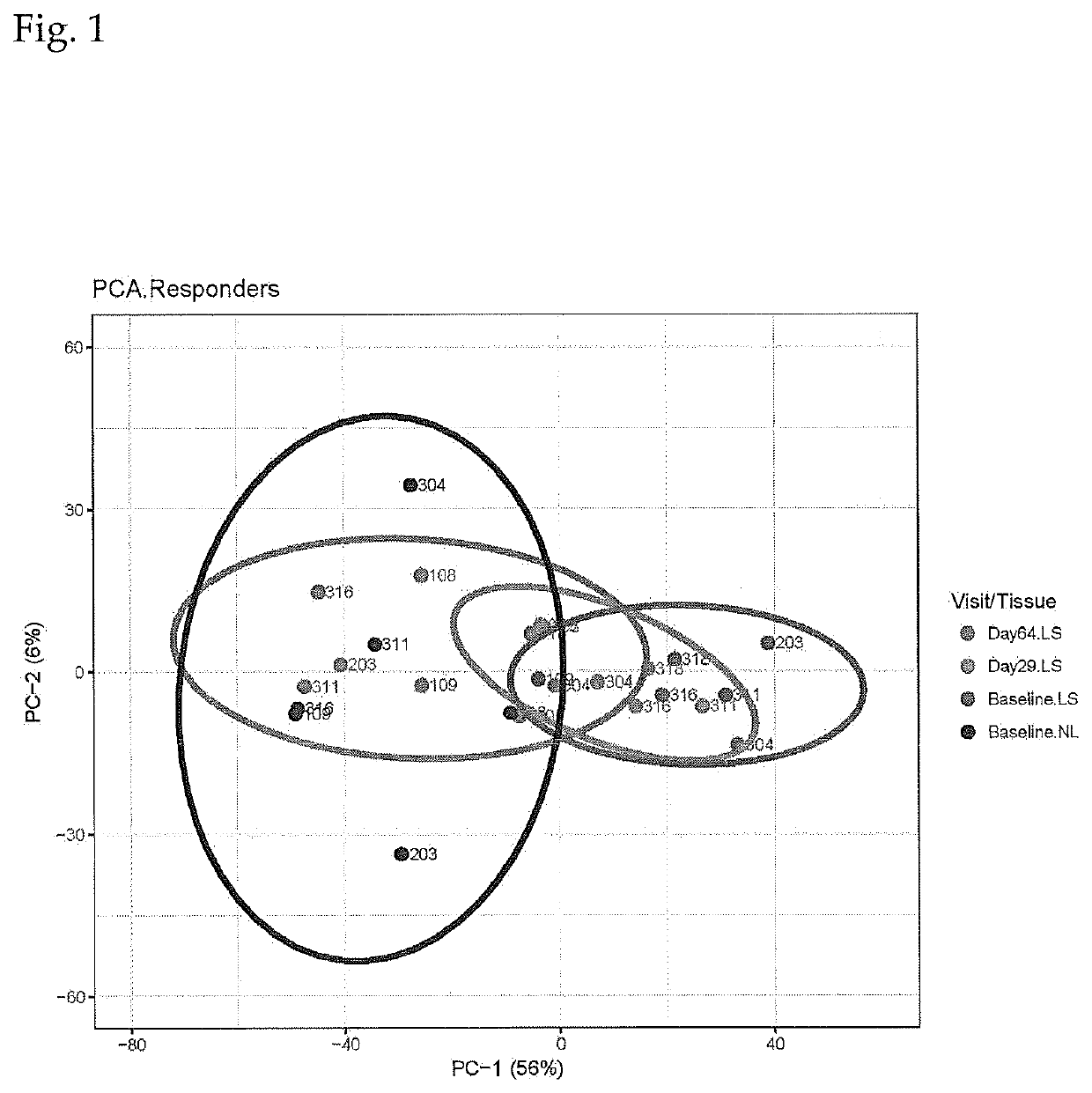
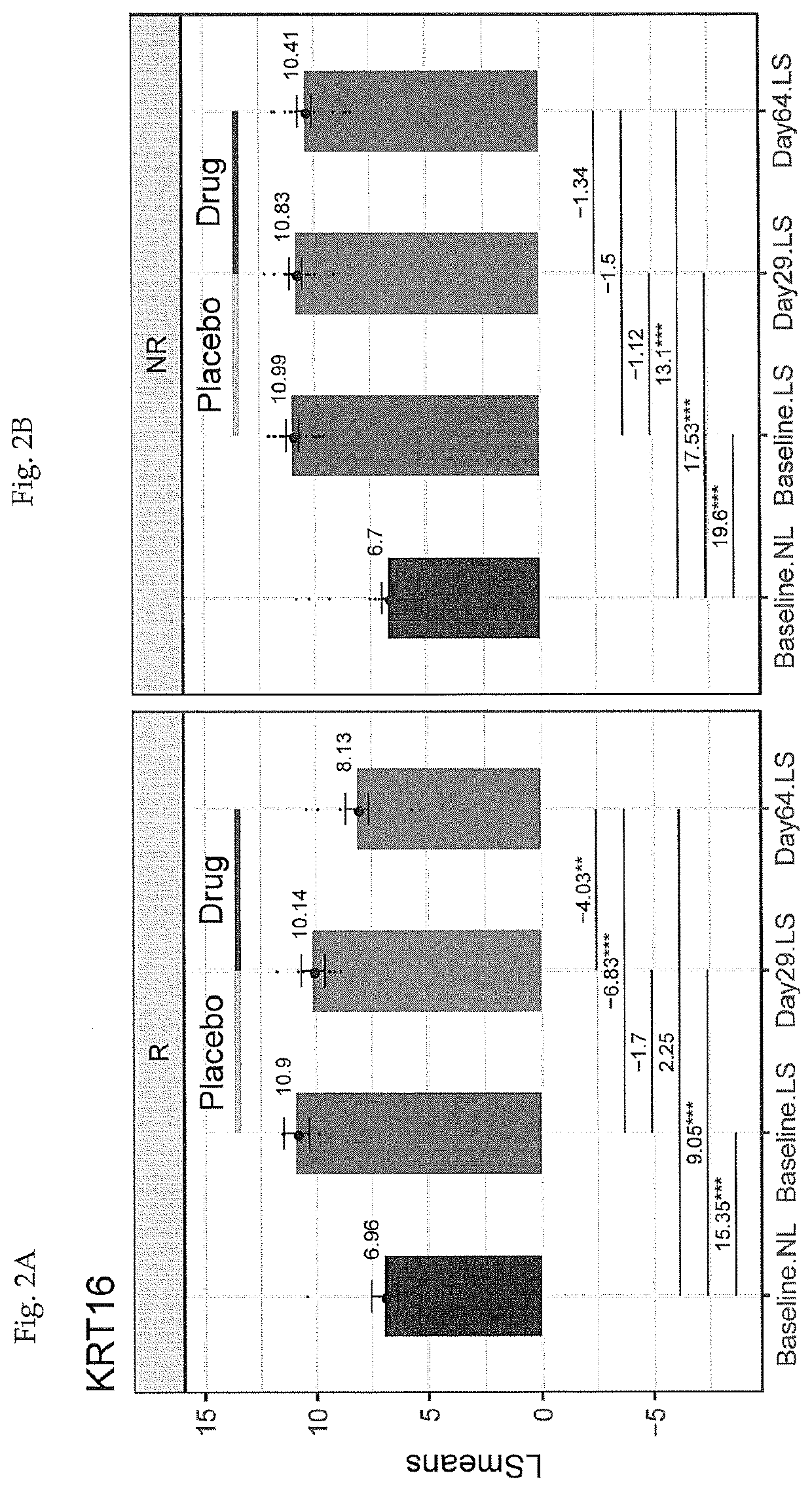
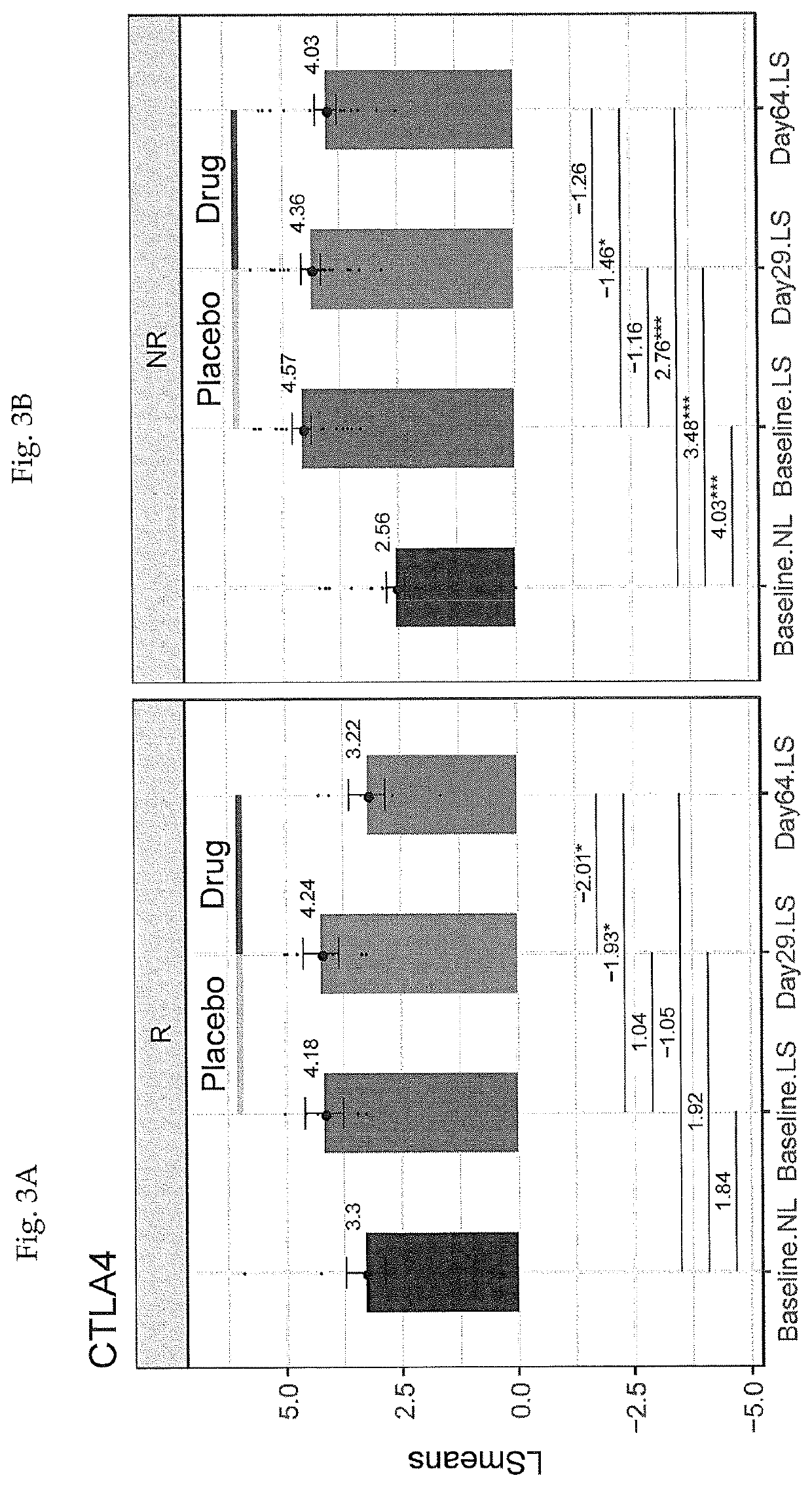
Combination Of Local And Systemic Therapies For Enhanced Treatment of Dermatologic Conditions
a local and systemic therapy and enhanced treatment technology, applied in the field of dermatology, can solve the problems of difficult management, difficult prediction and address, and challenge for interventions, and achieve the effect of boosting the therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044]The present invention contemplates a method for the treatment of a hyperproliferative skin disorder; i.e., an increased rate of skin cell turnover in the epidermis discussed and exemplified hereinafter, that comprises administration of a therapeutically effective amount of a topical halogenated xanthene pharmaceutical composition, in combination with a therapeutically effective amount of a systemic anti-inflammatory agent.
[0045]The present invention also particularly contemplates a method for the treatment of psoriasis and eczema, that comprises administration of a therapeutically effective amount of a halogenated xanthene pharmaceutical composition, in combination with a therapeutically effective amount of a systemic immune system down-regulating agent.
[0046]The non-clinical topical application studies of 14C-labelled rose bengal discussed hereinafter show that the rose bengal remains mostly in the stratum corneum, with decreasing amounts present in epidermis and dermis. No r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com