Nanocrystalline apatites and composites, prostheses incorporating them, and method for their production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis and Characterization of Nanocrystalline Hydroxyapatite
[0075]A nanocrystalline hydroxyapatite powder was successfully synthesized that allowed pressureless sintering without glassy sintering aids at a remarkably low temperature of 1100° C. for 2 hours or less, resulting in a material that was >98% dense.
[0076]A series of experiments were conducted to determine the feasibility of synthesizing nanocrystalline hdyroxyapatite and to determine the optimal pH, aging temperature, aging time, and heat treatment where the optimal hydroxyapatite is the sample that possesses the highest green and sintered densities. Reagent grade Ca(NO3)2.4H2O and (NH4)2HPO4 were used as starting materials. Aqueous solutins of (NH4)2HPO4 (NHP) and Ca(NO3)2(CaN) were prepared such that the Ca:P rate was 10:6. 0.300 M (NH4)2HPO4 and 0.500 M Ca(NO3)2 as well as 0.100 M (NH4)2HPO4 and 0.167 M Ca(NO3)2 were prepared. These solutions were mixed with a magnetic stirrer. The pH of the NH4)2 aqueous solution w...
example 2
Determination of Optimal Conditions—Calcination, and Comparison With Commercially-Available Hydroxyapatite Powder
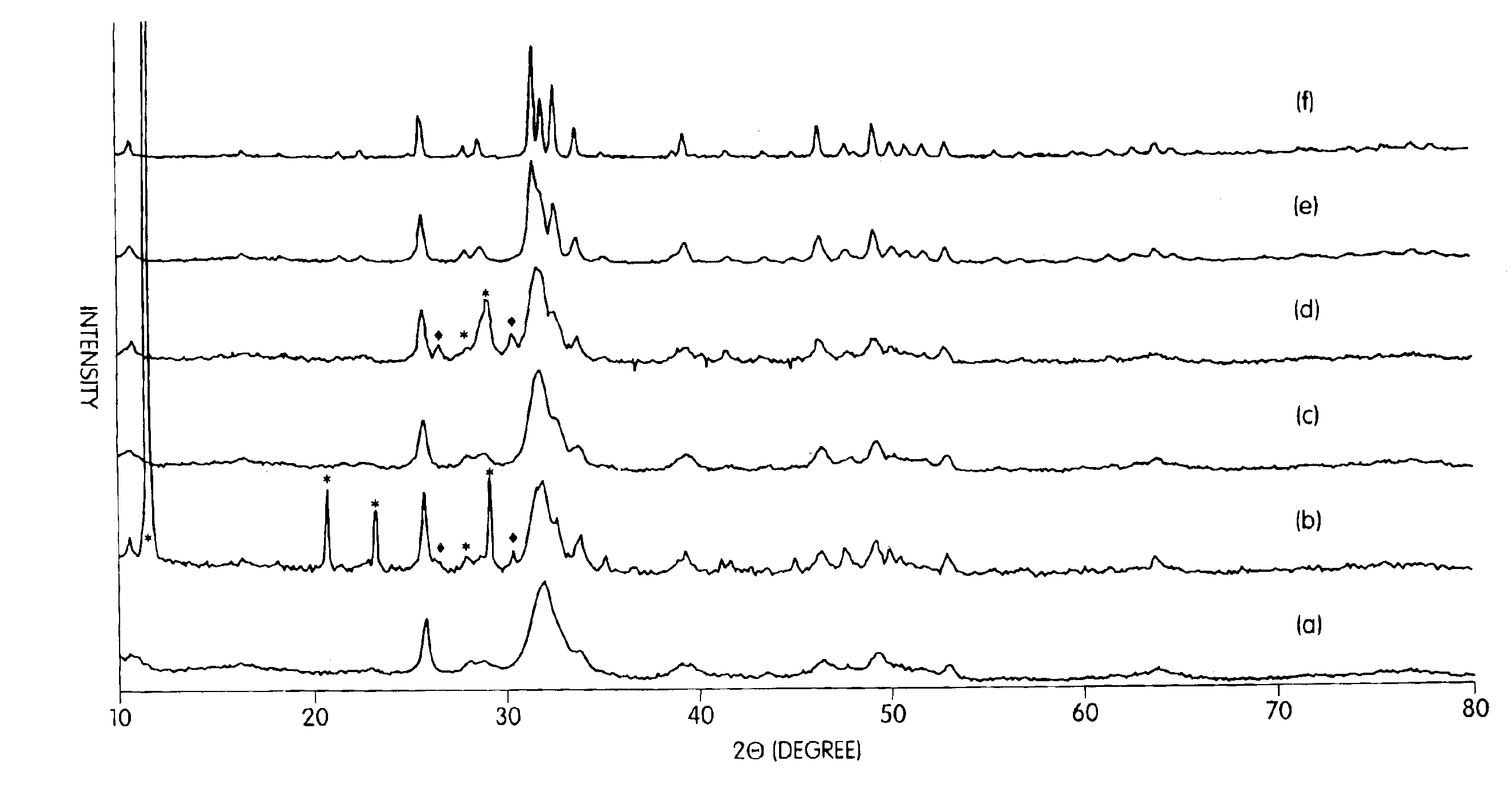
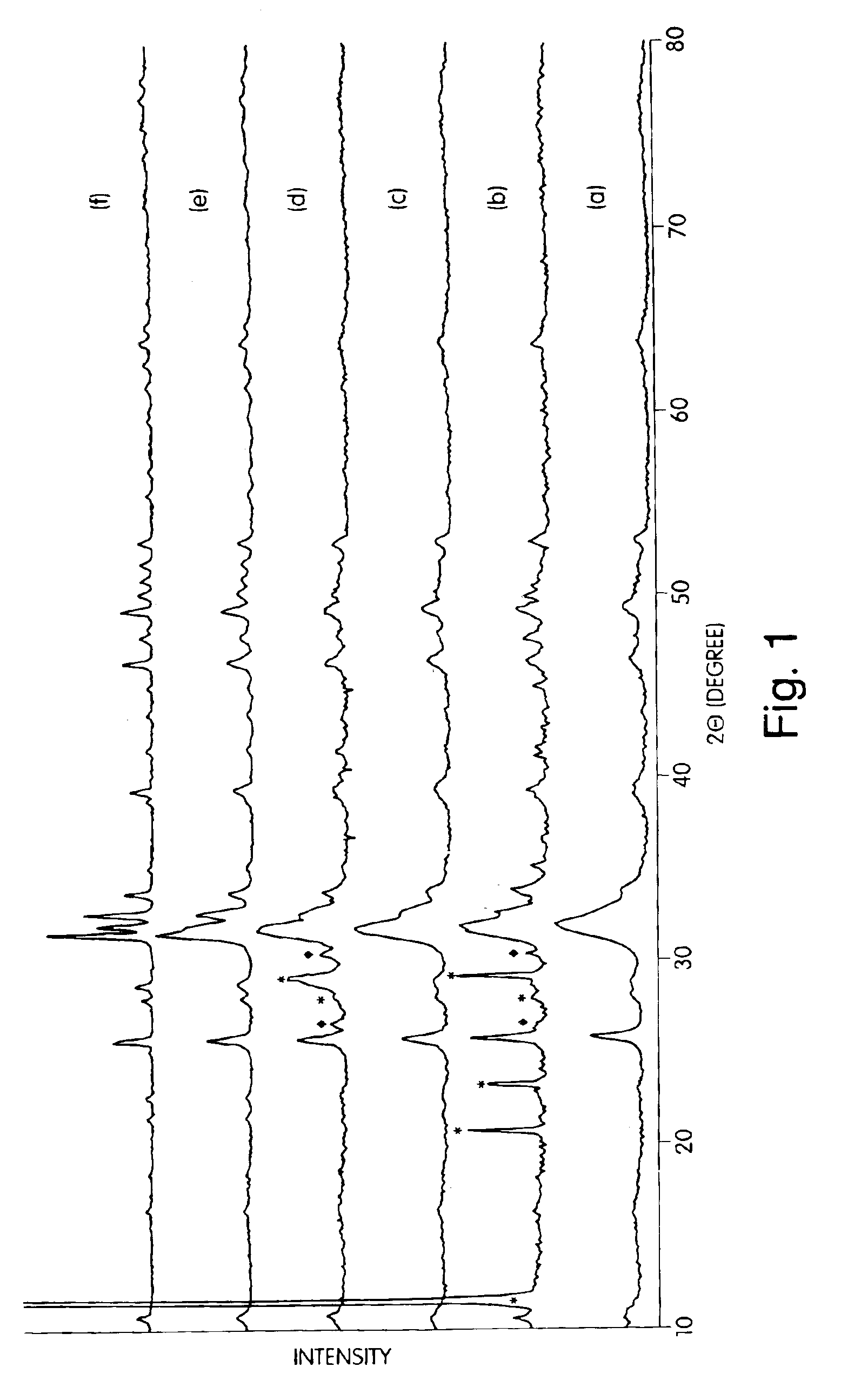
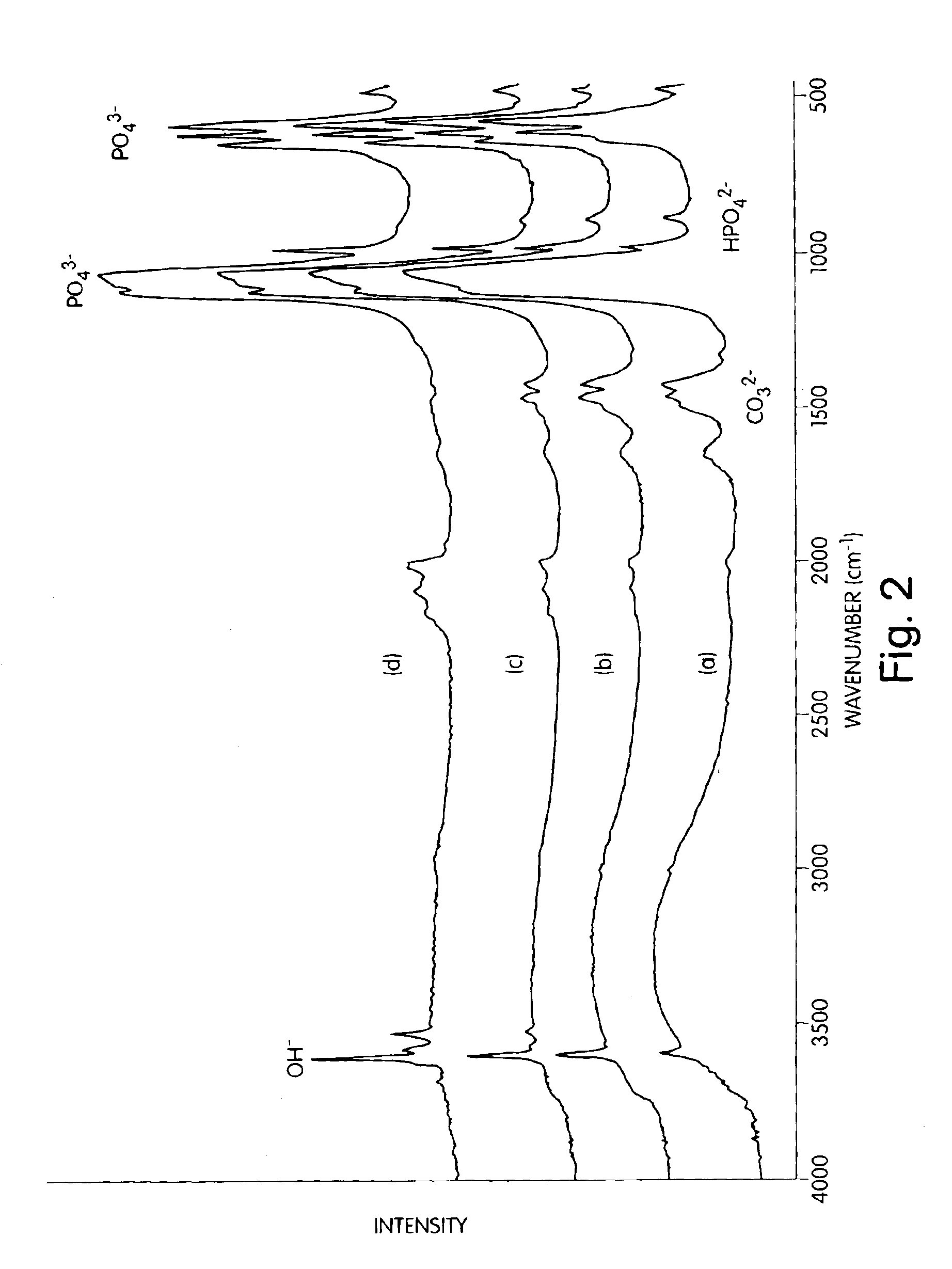
[0079]One sample of nanocrystalline hydroxyapatite from Example 1 (Trial 2) was heat-treated in air at 550° C., 700° C., and 900° C. for 2 hours in order to investigate the effect of calcination temperature on the microstructure of hydroxyapatite, Trial 2 synthesis conditionsa re presented in Table 1. The XRD patterns of the as-synthesized hydroxyapatite at various calcination temperatures (FIG. 1) indicated that the sample heat treated at 550° C. had better crystallinity than the precursor gel prior to the heat treatment, although the peaks were still quite broad. The heat treatment at 700° C. gave increased crystallinity compared to the sample treated at only 550° C. and was composed of only hydroxyapatite. Even after calcination at 900° C., the sample was found to be composed of only hydroxyapatite. The XRD patterns of the as-received conventional hdyroxyapatite powede...
example 3
Determination of Optimal Conditions—Sintering, and Comparison with Commercially-Available Hydroxyapatite Powder
[0084]The Trial 2 hydroxyapatite at 550° C. in air was CIPed and sintered at 1000° C., 1100° C., 1200° C. and 1300° C. in air. Conventional hydroxyapatite is known to be stable up to 1360° C. (K. De Groot, C. P. A. T. Klein, J. G. C. Wolker, and J. De Blicck-Hogervorst, “Chemistry of Calcim Phopshate Bioceramics,” Handbook of Bioactive Ceramics: Calcium Phosphate and Hydroxyapatite Ceramics, Vol 2, pp. 3-15, Edited by Yamamuro, L. L. Hench, and J. Wilson, CRC. Press, Boca Raton, 1990). The decomposition reaction is Ca10(PO4)6(OH)2→3Ca5(PO4)2+CaO+H2O and begins at 1200° C. (K. Kamiya, T. Yoko, K. Tanaka, Y. Fujiyama, “Growthof Fibrous Hydroxyapatite in Gel System, ” J. Mater. Sci., 24, 827-832, 1989). It has been reported that even below 1200° C. the loss of the OH− may occur (K. R. Venkatachari, D. Huang, S. P. Ostrander, W. Schulze, and G. C. Stangle, “Preparation of Nanoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com