Tetrahydro quinoline functional dye containing thiophene bridge chain
A tetrahydroquinoline and dye technology, applied in organic dyes, azo dyes, and analysis by chemical reaction of materials, etc., to achieve the effects of high fluorescence quantum yield, long excited state lifetime, and good chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
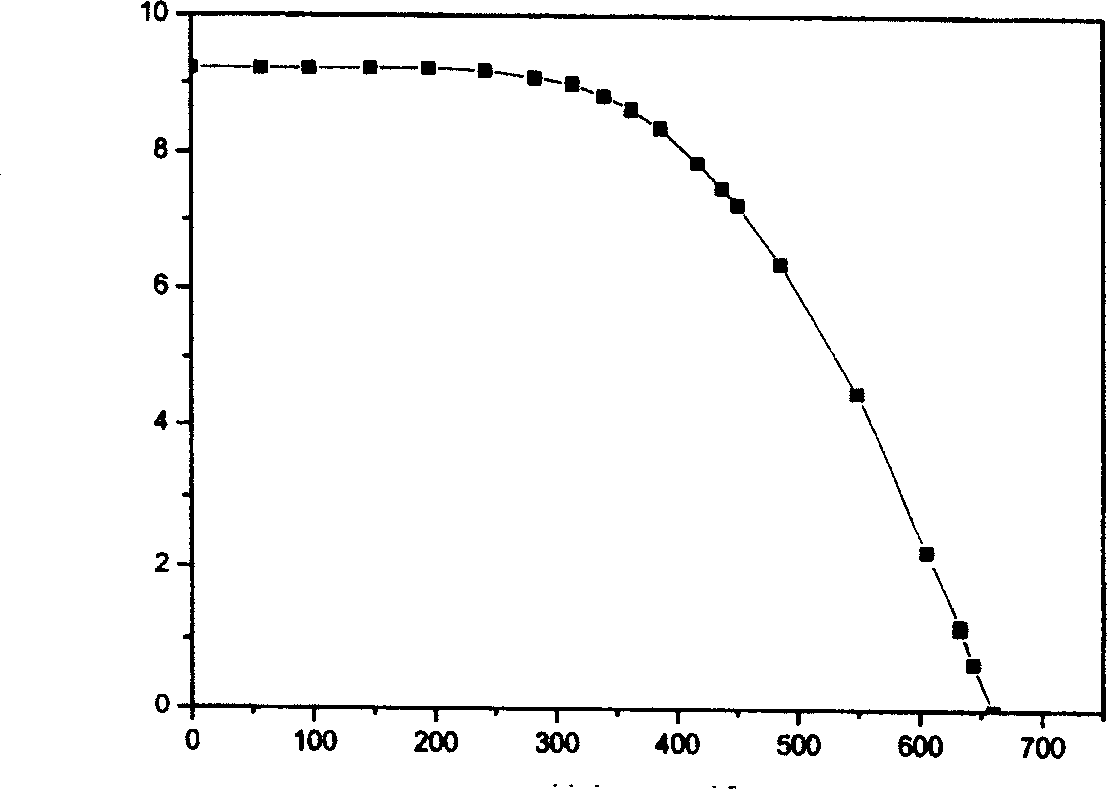
Image
Examples
Embodiment 1
[0029] Synthesis of 6-[5-(2-cyanoacrylate)thiophene-2-vinyl]-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline
[0030]Before each step of reaction, the experimental device was evacuated, and the instrument was preheated to drive off water vapor.
[0031] (1) Synthesis of 6-formyl-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline
[0032]
[0033] Add 1.7g (9mmol) 1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline and 3mL dry DMF into the flask under the protection of nitrogen, keep the temperature at 10-20°C In between, add 0.9mL of phosphorus oxychloride dropwise, heat to 50°C and keep warm for 3-4 hours after the dropwise addition; cool, add 50g of crushed ice cubes, stir well; add dilute sodium hydroxide solution to neutralize , a large amount of solid product was precipitated, filtered and dried to obtain compound 6-formyl-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline 1.3g (6mmol), yield 66.7% .
[0034] NMR 1 H-NMR (CDCl 3 , 400MHz, ppm): δ1.28(s, 3H), 1.34(s, 3H), 1....
Embodiment 2
[0056] Synthesis of 6-[5-(2-diethylphosphoacrylocyano)thiophene-2-vinyl]-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline
[0057]
[0058] 65mg (0.2mmol) 6-(5-formylthiophene-2-vinyl)-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline and 43mg (0.24mmol ) Diethyl cyanomethyl phosphate is added in the flask, acetonitrile is used as a solvent, and 6 to 8 drops of piperidine are added dropwise as a catalyst; heated to reflux for 3 hours, and the solvent is removed after reflux ends, and dichloromethane: ethyl acetate=15:1 The solvent of (v / v) is carried out column separation and purification on the silica gel column as eluent, obtains compound 6-[5-(2-diethyl phosphate acrylocyano) thiophene-2-vinyl]-1,2, 75 mg (0.15 mmol) of 2,4-tetramethyl-1,2,3,4-tetrahydroquinoline, yield 77.5%.
[0059] Melting point: 112-113°C;
[0060] NMR 1 H-NMR (Acetone-d 6 , 400MHz, ppm): δ1.24(s, 3H), 1.33(s, 3H), 1.34-1.39(m, 9H,), 1.47(dd, 1H), 1.87(dd, 1H), 2.80-2.89( m, 4H), 4.14-4.21(m, 4H),...
Embodiment 3
[0066] Synthesis of 6-{2-[5-(2-diethylphosphoacrylocyano)thienyl]}-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline
[0067] (1) Synthesis of 6-bromo-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline
[0068]
[0069]Dissolve 368mg (2mmol) of 1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline in 20mL carbon tetrachloride, add 392mg (2.2mmol) NBS and 185 mg (1.1 mmol) of azobisisobutyronitrile, heated to reflux, and the reaction was completed in 30 minutes, cooled, spin-dried, and used dichloromethane: n-hexane=1:3 (v / v) as eluent on a silica gel column After separation, 450 mg (1.68 mmol) of the product 6-bromo-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline was obtained, with a yield of 84.0%.
[0070] NMR 1 H-NMR (CDCl 3 , 400MHz, ppm): δ1.17(s, 3H), 1.25(s, 3H), 1.30(d, 3H,), 1.47(dd, 1H), 1.75(dd, 1H), 2.75-2.80(m, 4H), 6.40 (d, 1H), 7.15 (dd, 1H), 7.17 (s, 1H).
[0071] (2) Synthesis of 6-(2-thienyl)-1,2,2,4-tetramethyl-1,2,3,4-tetrahydroquinoline
[0072]
[0073] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com