Method for microwave synthesis of cerium fluoride nano disk
A technology of microwave synthesis and cerium fluoride, applied in chemical instruments and methods, nanotechnology, nanotechnology, etc., to achieve the effects of energy saving, uniform size and controllable size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
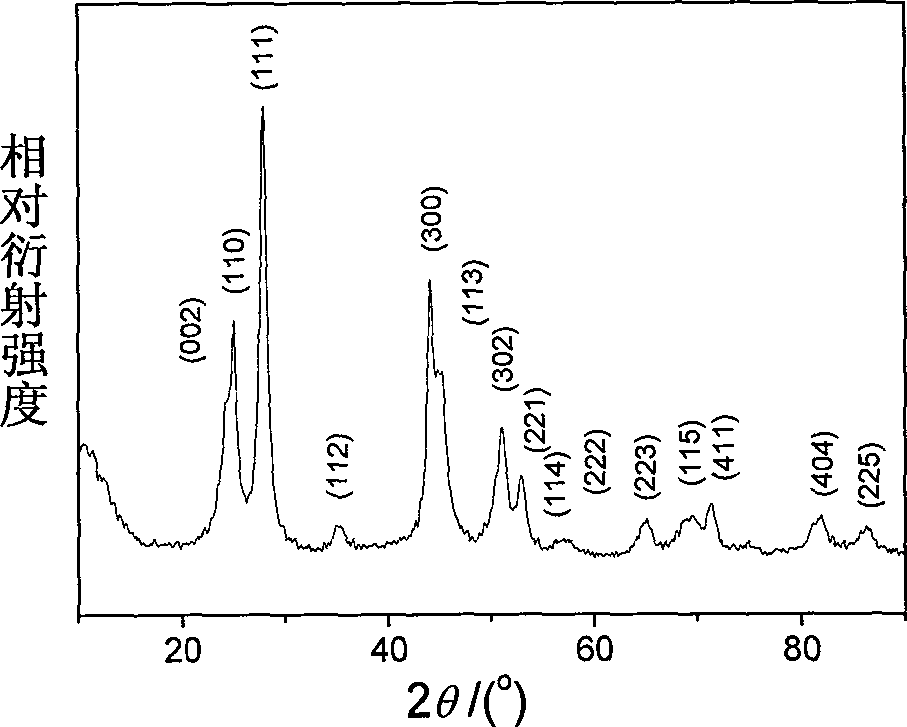
[0021] 4 mmol of disodium ethylenediamine tetraacetate (Na 2 h 2 EDTA) was dissolved in 100ml of deionized water, and a few drops of concentrated ammonia were added to dissolve it completely. Then add 2mmol cerium nitrate (Ce(NO 3 ) 3 ) to form a uniform solution. At this time, the concentration of cerium ions in the solution was 0.02M, and the molar ratio of complexing agent to cerium ions was 2.0:1; the pH value of the reaction system was adjusted to 6.0 with dilute nitric acid solution. Then add molar ratio KBF under vigorous stirring 4 / C 3+ Potassium fluoroborate is 4.0:1. The reaction solution was transferred into a round bottom flask, placed in a household microwave oven with a reflux device, heated for 30 minutes, and then the reaction system was naturally cooled to room temperature. The resulting product was collected and dried after centrifugation and deionized water washing to obtain cerium fluoride (CeF 3 )product. CeF 3 The X-ray diffraction pattern of th...
Embodiment 2
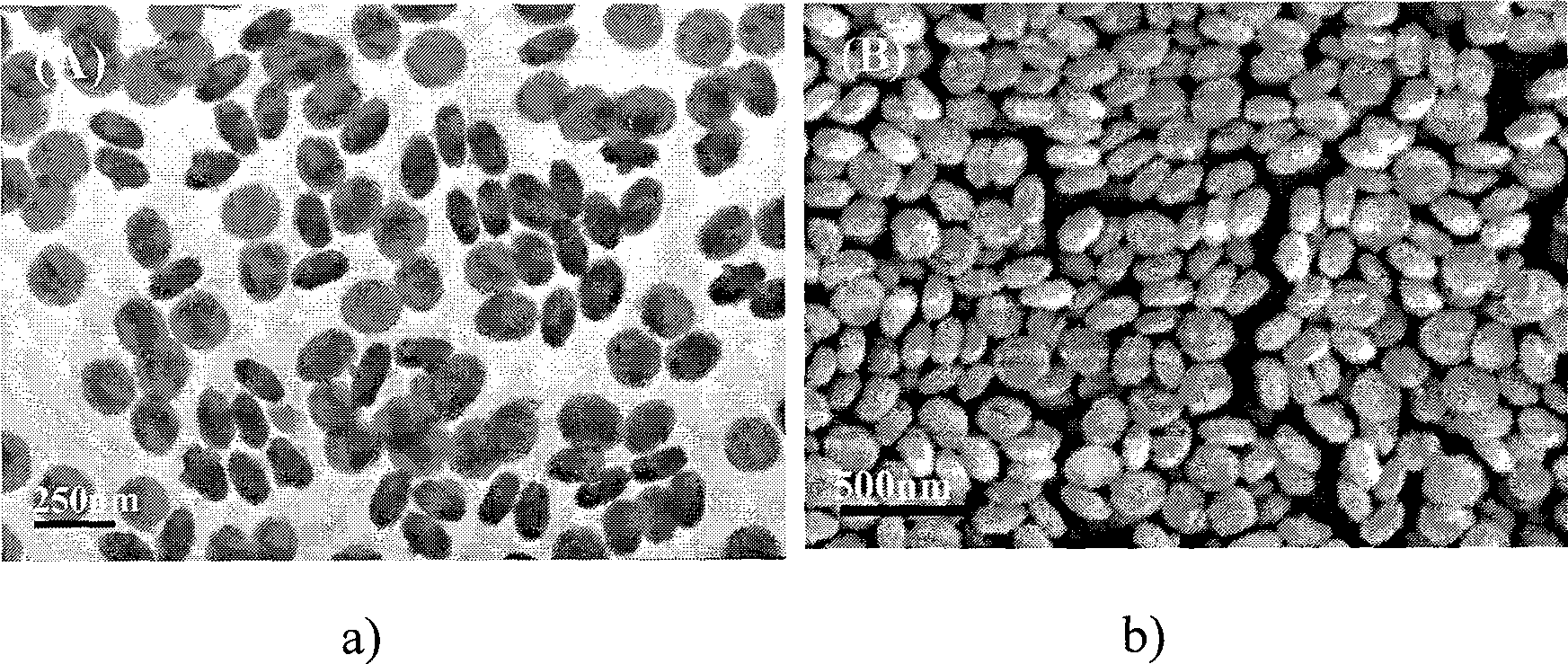
[0023] 4 mmol of disodium ethylenediamine tetraacetate (Na 2 h 2 EDTA) was dissolved in 100ml of deionized water, and a few drops of concentrated ammonia were added to dissolve it completely. Then add 2mmol cerium nitrate (Ce(NO 3 ) 3 ) to form a uniform solution. At this time, the concentration of cerium ions in the solution was 0.02M, and the molar ratio of complexing agent to cerium ions was 2.0:1; the pH value of the reaction system was adjusted to 6.0 with dilute nitric acid solution. Then add molar ratio KBF under vigorous stirring 4 / C 3+ 4.0:1 potassium fluoroborate (KBF 4 ). The reaction solution was transferred into a round bottom flask, placed in a household microwave oven with a reflux device, heated for 60 minutes, and then the reaction system was naturally cooled to room temperature. The resulting product was collected by centrifugation and washed thoroughly with deionized water and dried to obtain CeF 3 product. CeF 3 The TEM and SEM images of the prod...
Embodiment 3
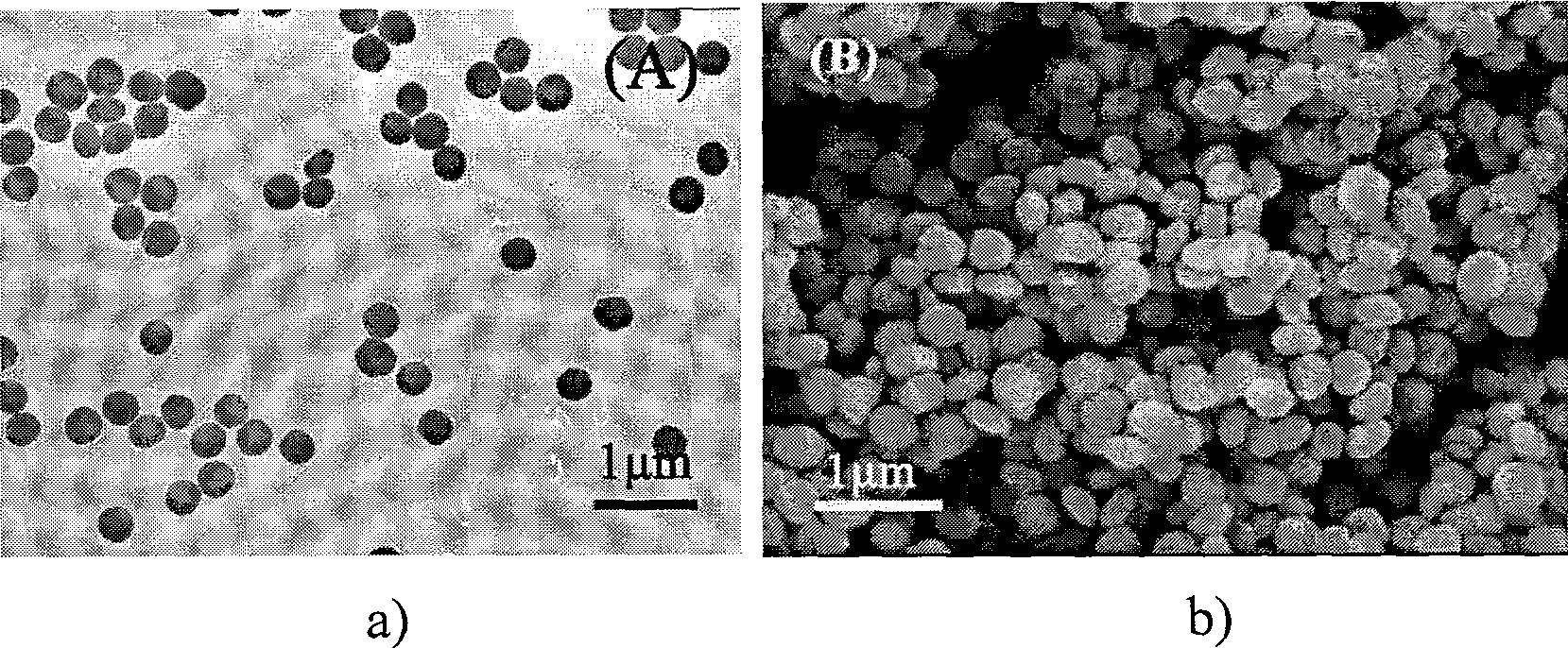
[0025] 4 mmol of disodium ethylenediamine tetraacetate (Na 2 h 2 EDTA) was dissolved in 100ml of deionized water, and a few drops of concentrated ammonia were added to dissolve it completely. Then add 2mmol cerium nitrate (Ce(NO 3 ) 3 ) to form a uniform solution. At this time, the concentration of cerium ions in the solution was 0.02M, and the molar ratio of complexing agent to cerium ions was 2.0:1; the pH value of the reaction system was adjusted to 6.0 with dilute nitric acid solution. Then add molar ratio KBF under vigorous stirring 4 / C 3+ Potassium fluoroborate is 4.0:1. The reaction solution was transferred into a round bottom flask, placed in a household microwave oven with a reflux device, heated for 45 minutes, and then the reaction system was naturally cooled to room temperature. The resulting product was collected by centrifugation and washed thoroughly with deionized water and dried to obtain CeF 3 product. TEM and SEM observations revealed that CeF 3 It...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com