Method of producing alpha- terpineol
A technology of terpineol and petroleum ether, applied in the field of preparation of alpha-terpineol, can solve the problems of less reuse, equipment corrosion, long time consumption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
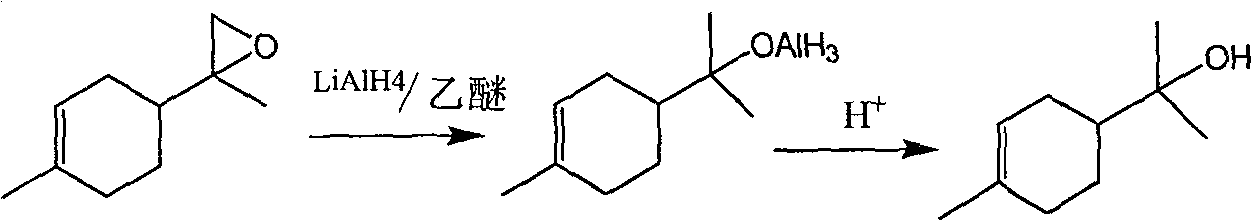
[0012] The preparation method of α-terpineol comprises the following steps: a preparation method of α-terpineol comprises the following steps:
[0013] a. Add lithium aluminum hydride to the organic solvent at 0°C, dissolve 8,9-epoxy limonene in the organic solvent, add 8,9-epoxy limonene solution dropwise to the lithium aluminum hydride solution, Carry out the ring-opening reaction at 0-5°C for 5-10 hours, add ice water drop by drop to the reaction system under stirring until no hydrogen is released, then add 5-20% dilute sulfuric acid to the reaction system to dissolve The aluminum hydroxide produced by the reaction precipitates, separates the water layer, extracts with ether, washes with saline solution, and filters to remove the ether to obtain a crude product, wherein the organic solvent is selected from ether, tetrahydrofuran, N,N-dimethylformamide or di One of methyl chloride; b. The crude product is separated with a silica gel column, and the product is rinsed with a m...
Embodiment 1
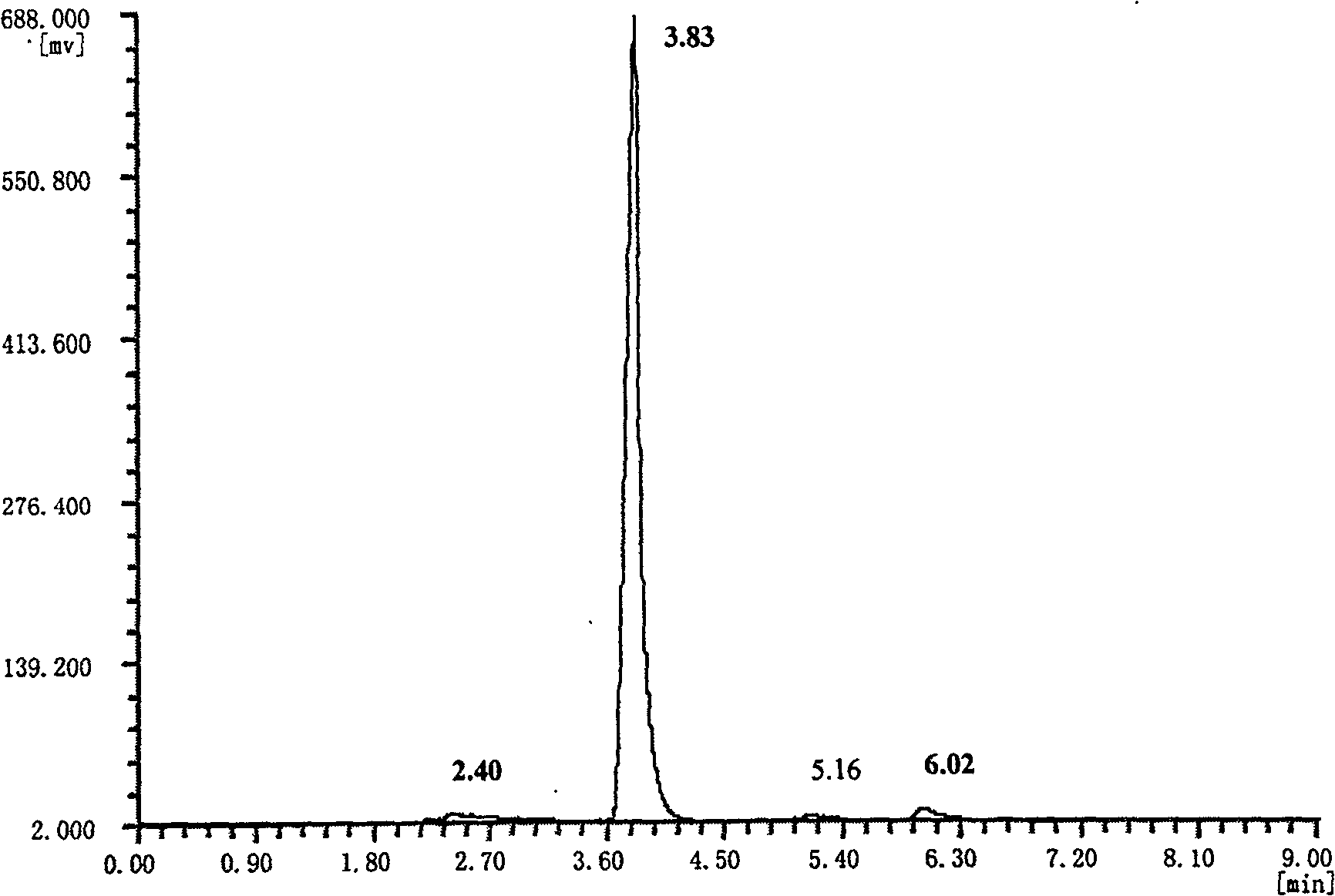
[0015] Weigh 0.9865 g of lithium aluminum hydride into a 100 mL three-necked flask filled with 30 mL of anhydrous ether, and at 0°C, add 1.0148 g of 8,9-epoxylimonene and 10 ml of anhydrous ether solution dropwise, and simultaneously turn on the magnetic Stirrer, after the raw material is added dropwise, react at room temperature for 6.5 hours. Cool the product obtained by the reaction with ice water, and add ice water very carefully (drop by drop) under stirring until no hydrogen is released, and then add 25 ml of 10% sulfuric acid by mass fraction to precipitate aluminum hydroxide was dissolved. Separate the water layer with a separatory funnel, and extract three times with ether, combine the ether extracts, wash with saturated saline solution, add Na 2 SO 4 Drying, filtration and evaporation of ether gave 0.6316 g of product. Such as figure 1 Shown, the terpineol purity of the present invention is greater than 98% as determined by gas chromatography.
[0016] Table 1 g...
Embodiment 2
[0024] Weigh 0.5034 grams of lithium aluminum hydride and pour it into a 100 mL three-necked flask filled with 30 ml of anhydrous tetrahydrofuran, cool it with ice water and stir it magnetically, add dropwise a mixture of 0.5011 grams of 8,9-epoxylimonene and 10 ml of anhydrous tetrahydrofuran solution, reacted at room temperature for 6.5 hours. After the reaction was finished, cool with ice water, add ice water dropwise under stirring until no more hydrogen gas was released, then add 25ml of sulfuric acid with a mass fraction of 10%, and the aluminum hydroxide precipitate was dissolved. Separate with a separatory funnel, extract the aqueous layer three times with ether, combine the ether solutions and wash with saturated brine, add Na 2 SO 4 Drying, filtration and evaporation of ether gave 0.2985 g of product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com