Dripping pills of schefflera arboricola, and its preparing method
A technology of Aescinus chinensis and dripping pills, which is applied in pill delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc. It can solve the problems of affecting the full effect of the medicine, low bioavailability, unstable quality, etc., and achieves benefits Labor protection and environmental protection, high bioavailability, good drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Take 3000g of horse chestnut leaves, add water and decoct twice. The amount of water added is 8 times the amount for the first time and 6 times the amount for the second time. The decoction time is 3 hours for the first time and 2 hours for the second time. Combine the decoction, let it stand for 12 hours, filter, and concentrate the filtrate to an extract with a relative density of 1.12~1.22 (60℃). Add ethanol to make the alcohol content 60%. Stir evenly, let stand for 12 hours, and take the supernatant Recover ethanol and concentrate it to an extract with a relative density of 1.15~1.25 (60℃). Add ethanol to make the alcohol content 70%. Stir it evenly. Let it stand for 12 hours. Take the supernatant to recover the ethanol and concentrate it into the extract. The extract is vacuum dried to obtain an extract. Take the extract, crush, and pass through a 100-mesh sieve. The weight ratio is horse chestnut extract: polyethylene glycol 6000 =1:2 ratio, add it to the ...
Embodiment 2
[0017] Example 2: Take the extract of Example 1, pulverize, and pass through a 100-mesh sieve. According to the weight ratio, the ratio of horse chestnut extract: mixed matrix = 1:1.5 (the mixed matrix is polyethylene glycol 6000 :Poloxamer according to the formula of 1:0.3), add it to the molten mixed matrix (82℃), stir evenly, drop into the simethicone coolant (3℃), separate the dripping pills, absorb and cool Agent, dry, ready.
Embodiment 3
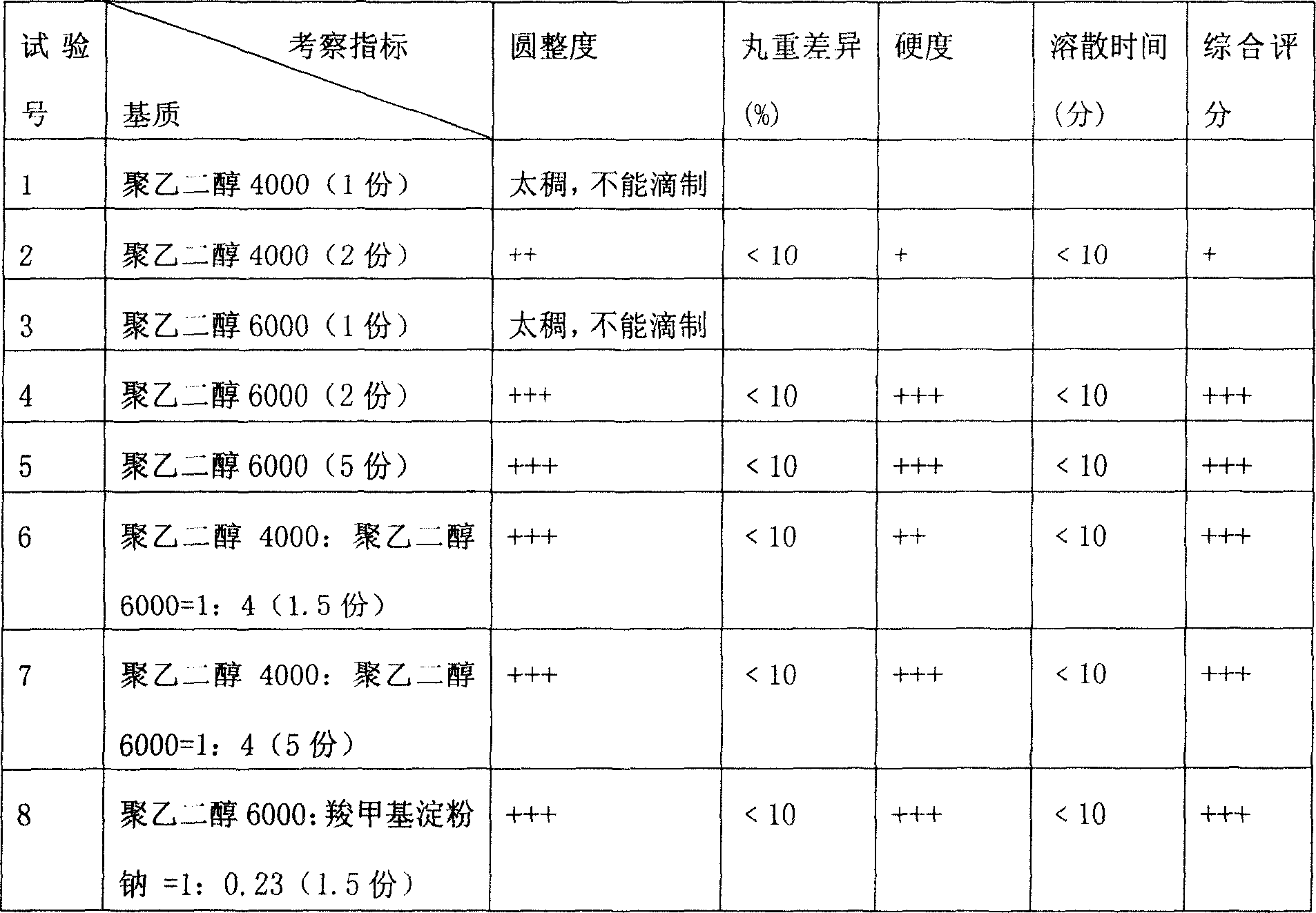
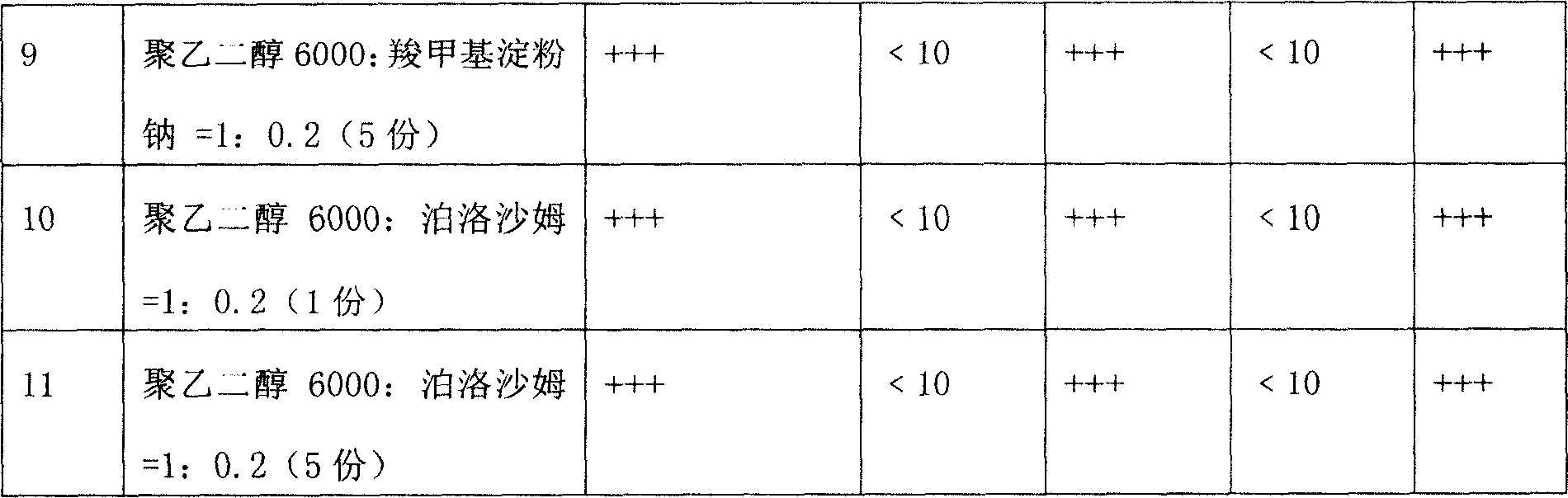
[0018] Example 3: In this example, through the formulas of horse chestnut extract and different substrates, indicators such as roundness, pill weight difference, hardness, and dissolution time were investigated to determine the weight ratio of the extract to the single or mixed substrate.
[0019] Horse chestnut extract and different matrix formula test (extracts are 1 copies)
[0020] Note: 1. The coolant is dimethyl silicone oil or liquid paraffin, and the cooling temperature is 1~5℃; the temperature of the medicine material and dripper is 80~85℃; the dripping speed is 30~50 particles / min.
[0021] 2. The above results show that all the indexes of test No. 4-11 are good, that is, when the ratio of extract to substrate is 1:1 to 1:5, it can be dripped smoothly. However, considering the dosage and other factors, the preferred ratio is Test No. 4, 6, 8, and 10, that is, the ratio of extract to matrix is 1:1 to 1:2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com