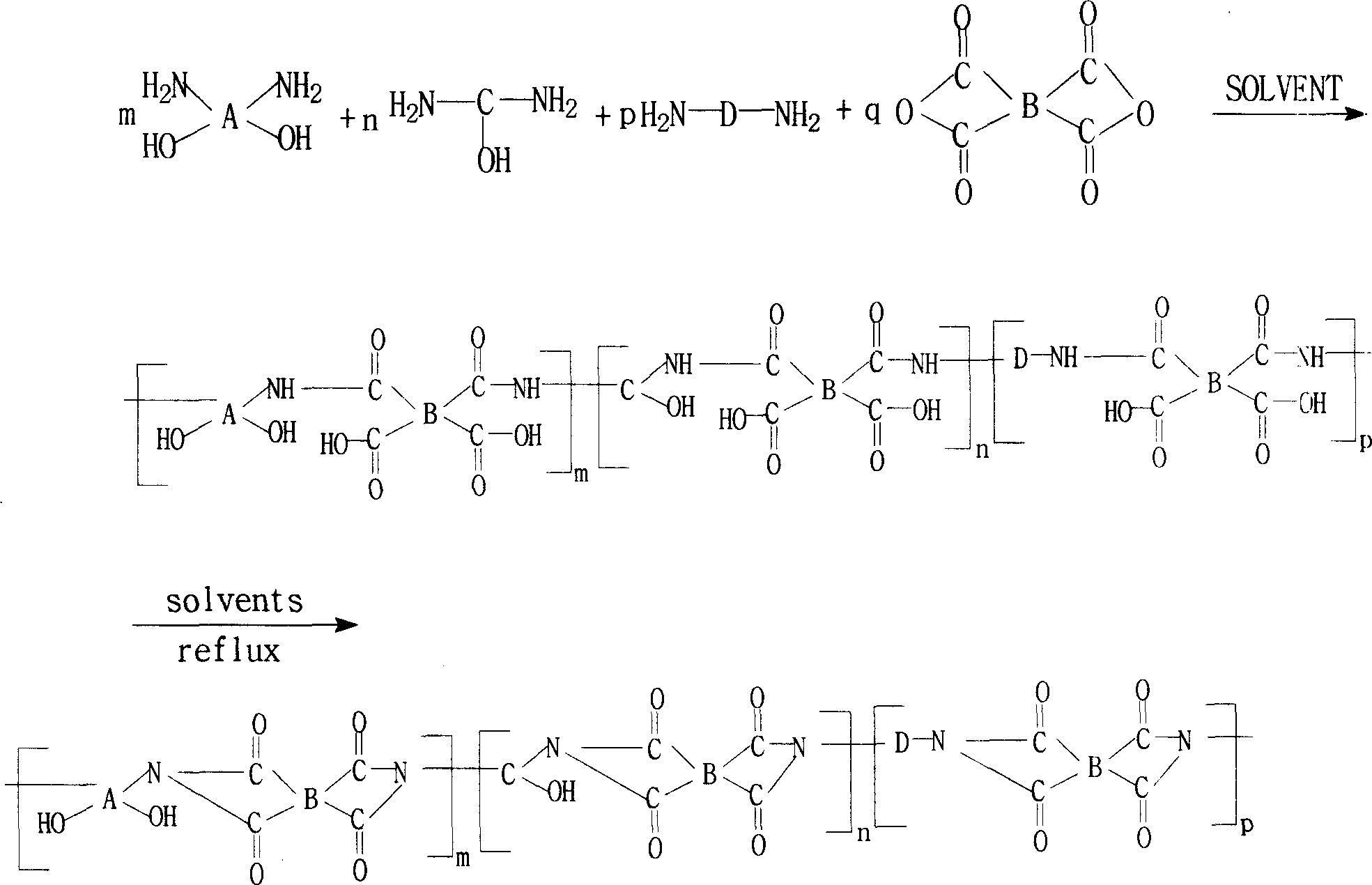
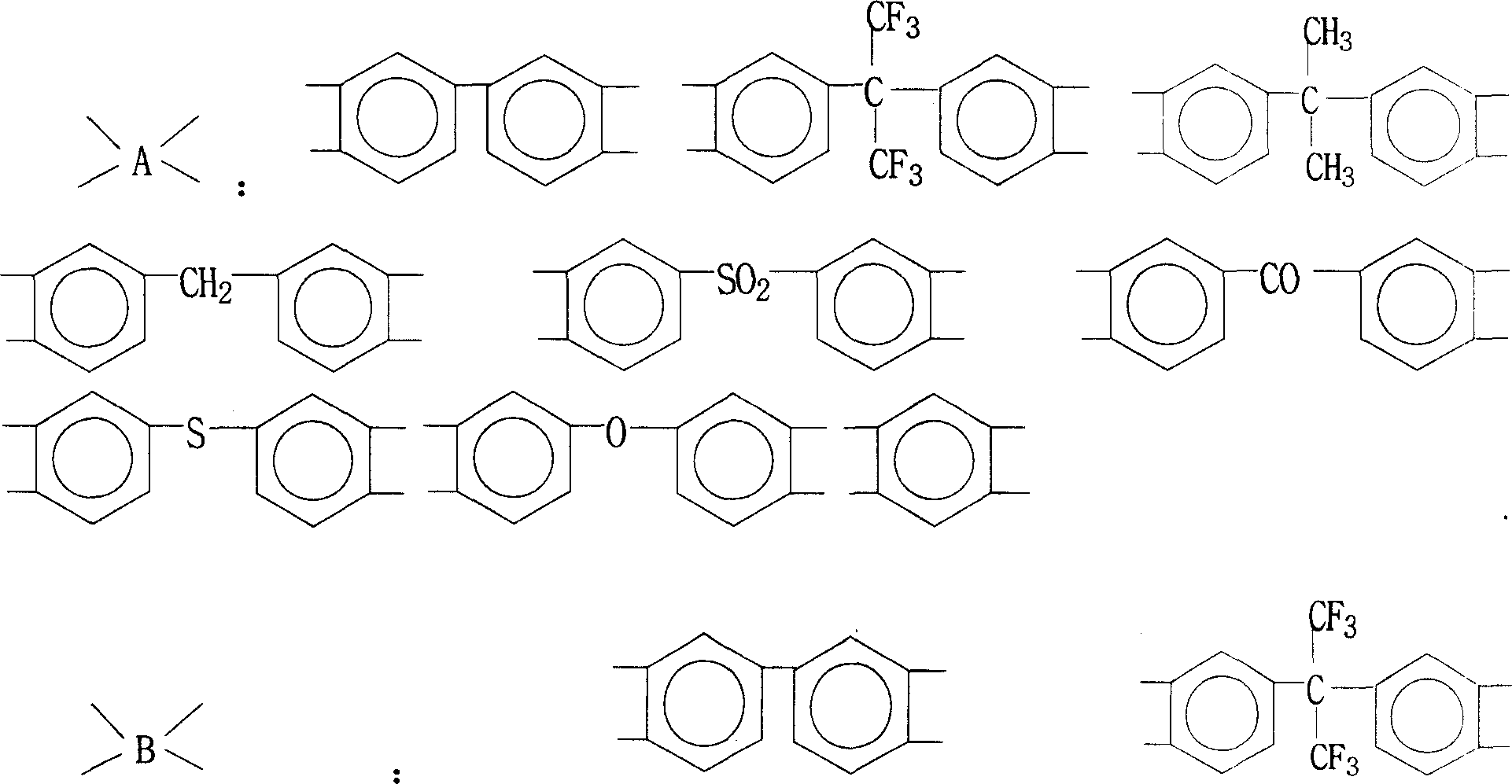
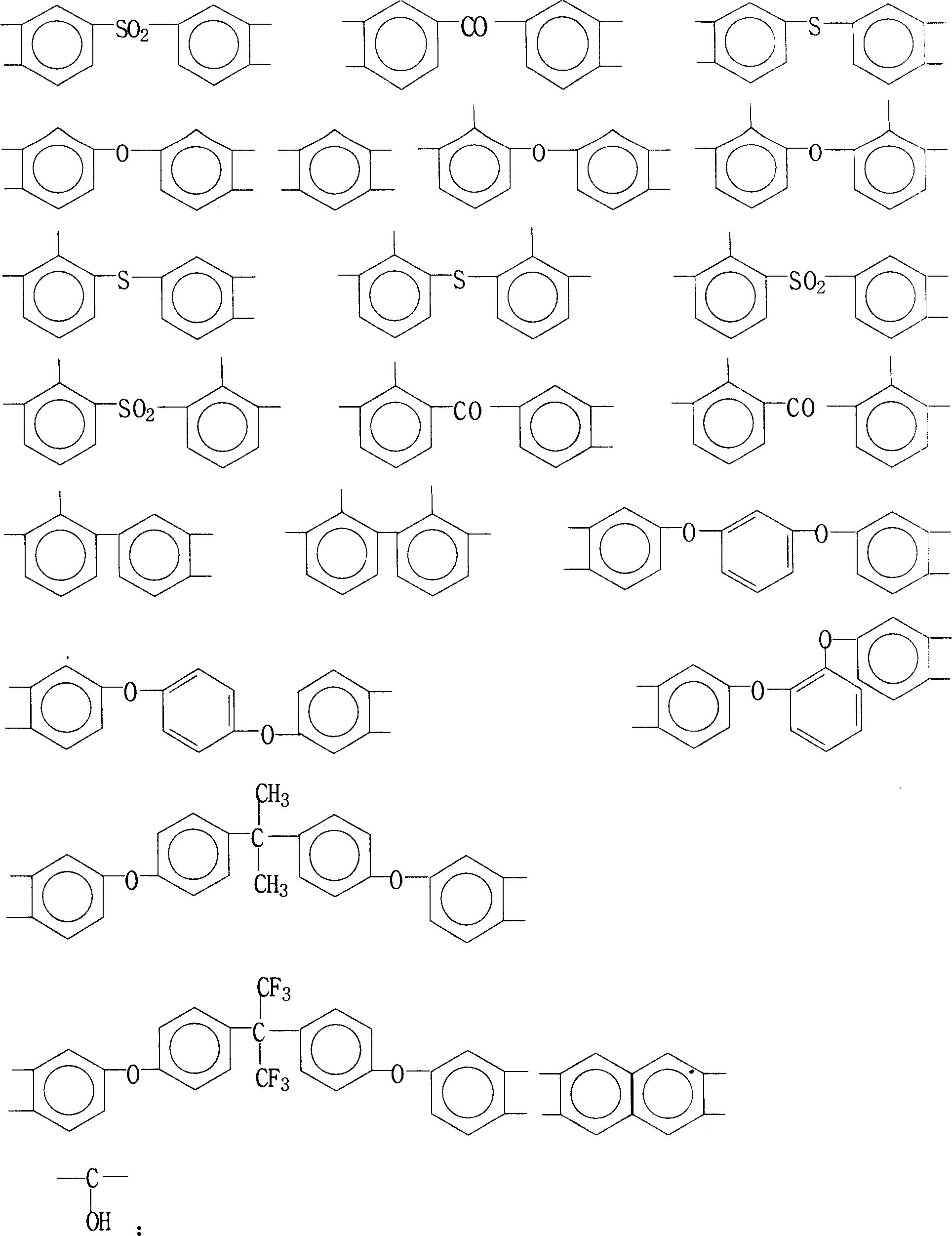Method for preparing binder of polyimide of containing phenolic hydroxyl group
A technology of phenolic hydroxyl polyimide containing phenolic hydroxyl groups, which is applied in the field of chemical polymer adhesive preparation, which can solve the problems of decreased heat resistance of the adhesive system, restrictions on the use of printed circuit boards, and copper foil oxidation reactions. , to achieve the effect of improving the bonding strength, improving the oxidation resistance and reducing the curing temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 3.66 g (0.01 mol) of 2,2-bis(3-amino-4-hydroxyphenyl)-1,1,1,3,3,3-hexafluoropropane (B3A4HPFP), 2.26 g (0.01 mol) of 3 , 3'-dimethyl 4,4'-diaminodiphenylmethane (DMMDA) and 5.85 grams (0.02 moles) of 1,3-bis(4-aminophenoxy)benzene (134BAPB) were added to the oil-water separation Then add 100 grams of N,N-dimethylacetamide (DMAc), stir at room temperature, make it dissolve completely, and cool in an ice-water bath To below 5°C, add 6.20 grams (0.02 moles) of 3,3',4,4'-tetracarboxylic diphenyl ether dianhydride (ODPA) and 6.44 grams (0.02 moles) of 3,3',4,4' -Tetraformic acid benzophenone dianhydride (BTDA), after stirring and reacting at 0° C. to 10° C. for 4 hours, a viscous polyhydroxyamic acid solution was obtained. Subsequently, nitrogen gas was introduced, 20 grams of toluene was added, and the temperature was raised to 120°C. After reflux and water separation for 2 hours, the heating system was turned off, stirred, and naturally cooled to room temperature to obtai...
Embodiment 2
[0034] 2.90 grams (0.01 moles) of 4,4'-diamino-4"-hydroxytriphenylmethane (DAHTM), 2.00 grams (0.01 moles) of 3,4'-diaminodiphenyl ether (34ODA), 5.18 grams (0.01 mol) 2,2-bis[4-(4-aminophenoxy)phenyl]hexafluoropropane (4BAPOFP), 2.92 g (0.01 mol) 1,3-bis(4-aminophenoxy)benzene (134BAPB ) into the reaction flask with oil-water separator, reflux condenser, thermometer, nitrogen pipe and electric stirrer, then add 100 grams of N-methyl-2-pyrrolidone (NMP), stir at room temperature, make it completely After dissolving, cool in an ice-water bath to below 5°C, add 5.20 grams (0.01 moles) of bisphenol A diether dianhydride (BPADA), 6.44 grams (0.02 moles) of 3,3',4,4'-tetracarboxylic acid Benzophenone dianhydride (BTDA) and 2.49 grams (0.01 mole) biphenyl dianhydride (BPDA), after 8 hours of stirring reaction at 0 ℃~10 ℃, obtain the viscous polyhydroxyamic acid solution. Subsequently, Introduce nitrogen, add 60 grams of xylene, heat up to 140°C, reflux and separate water for 1 hour...
Embodiment 3
[0038] 3.66 grams (0.01 moles) of 2,2-bis(3-amino-4-hydroxyphenyl)-1,1,1,3,3,3-hexafluoropropane (B3A4HPFP), 4.10 grams (0.01 moles) of 2 , 2-bis[4-(4-aminophenoxy)phenyl]propane (BAPOPP), 2.00 g (0.01 mole) 3,4'-diaminodiphenyl ether (34ODA) and 2.00 g (0.01 mole) 4 , 4'-diaminodiphenyl ether (44ODA) was added to the reaction flask with oil-water separator, reflux condenser, thermometer, nitrogen pipe and electric stirrer, followed by adding 120 grams of N, N-dimethyl formaldehyde Amide (DMF), stirred at room temperature, after making it completely dissolved, cooled in an ice-water bath to below 5°C, and added 9.30 grams (0.03 moles) of 3,3',4,4'-tetracarboxylic diphenyl ether dianhydride ( ODPA) and 3.22 grams (0.01 mole) of 3,3',4,4'-tetracarboxylic acid benzophenone dianhydride (BTDA), after stirring and reacting at 0°C to 10°C for 6 hours, a viscous poly Hydroxyamic acid solution. Subsequently, nitrogen gas was introduced, 20 grams of toluene was added, the temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com