Scale preparation method of bursin and application of used as avian influema vaccine adjuvant
A technology of tripeptide bursin and avian influenza, applied in the fields of immunology, veterinary medicine and veterinary medicine, can solve the problems of cost reduction, low tripeptide bursin activity, cumbersome steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
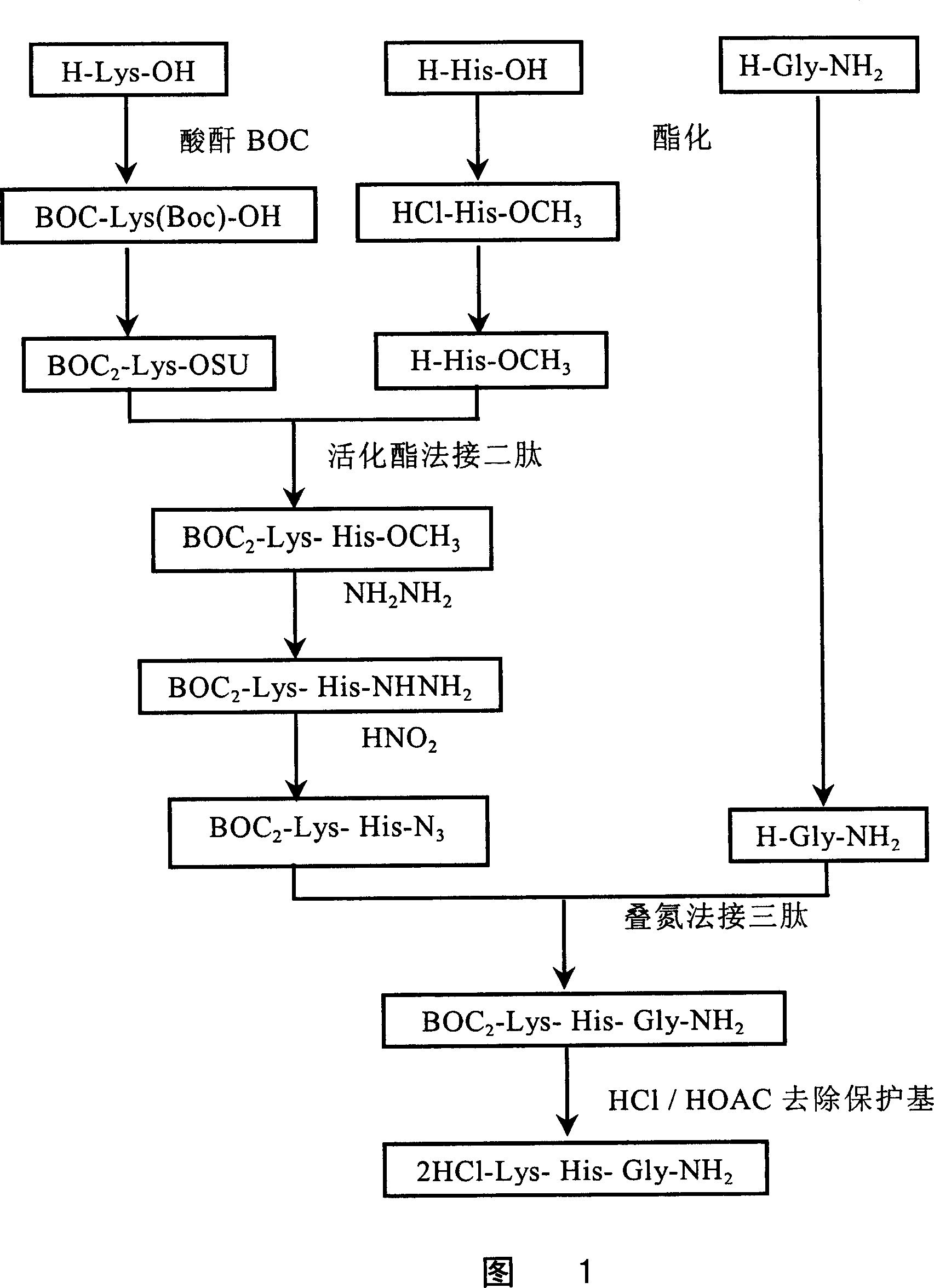
[0136] Combination method of azide and activated ester:
[0137] See Figure 1. The steps are as follows:
[0138] 1. Preparation of BOC-Lys(BOC)-OSU:
[0139] Get 100 grams of L-Lys-OH (684 mg molecules) and dissolve it in 800 milliliters of 1N NaOH solution, add dropwise 700 milliliters of tert-butanol solution containing 340 grams of BOC anhydride at room temperature, and maintain the reaction solution pH8-9 with 4N NaOH aqueous solution, Share 400 ml of 4N NaOH and react overnight at room temperature. The next day, the reaction solution was cooled in an ice bath, adjusted to pH 4 with 1N HCl, extracted three times with ethyl acetate, combined with ethyl acetate layers, washed with 5% NaCl aqueous solution to neutral pH, dried over anhydrous sodium sulfate, filtered The desiccant was removed, and the ethyl acetate was removed under reduced pressure to obtain 215 g of a white oil with a yield of 91%.
[0140] Weigh 103.9 grams of BOC-Lys(BOC)-OH (300 millimoles) and disso...
Embodiment 2
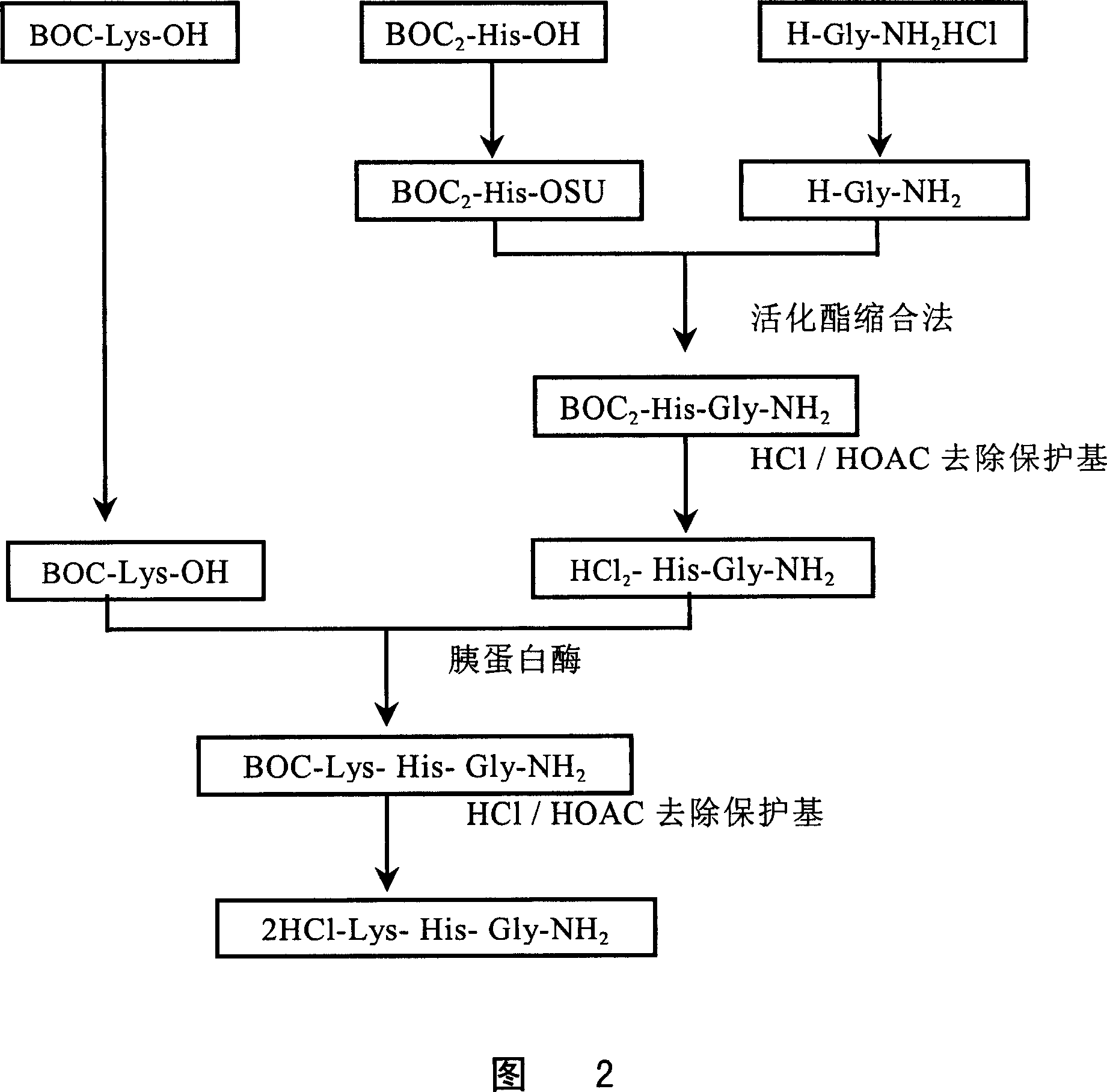
[0161] enzymatic synthesis
[0162] See Figure 2. The steps are as follows:
[0163] 1. Preparation of BOC-His(BOC)-OSU:
[0164] Weigh 106.6 g of BOC-His(BOC)-OH (300 mg) and dissolve it in 500 ml of THF, add 34.5 g of HOSU (300 mg) to completely dissolve, cool the salt to below -5°C in an ice bath, and add dropwise 61.8 gram of DCCI in THF, and the reaction solution was placed in the refrigerator overnight after the addition. The next day, the DCU solid was filtered off, the filtrate was concentrated to dryness under reduced pressure, 150 ml of hot isopropanol was added to dissolve the residue, and after cooling, 500 ml of n-hexane was added to precipitate a solid, which was collected by filtration to obtain 113.4 g, with a yield of 83.6 %, melting point: 66.2-68°C.
[0165] 2. BOC-His(BOC)-Gly-NH 2 preparation:
[0166] Weigh 112.2 g of BOC-His(BOC)-OSU (248 mg) and dissolve in 300 ml of THF, add 27.2 g of HCl-Gly-NH 2 (248 mg molecules), after mixing, slowly add 68 ...
Embodiment 3
[0177] Tripeptide bursin has a significant adjuvant function for H5 and H9 avian influenza vaccines (H5 is a highly pathogenic avian influenza virus vaccine):
[0178] In this example, the avian influenza vaccine was selected for research. 480 one-day-old Xugang yellow chickens were purchased from Foshan Xuganghuang Animal Husbandry Co., Ltd.
[0179] Animals were randomly grouped as shown in Table 2 below, and the chicks were adapted for one week after they were bought back, and they were vaccinated.
[0180] BS / weight
μg / Kg
BS dosage (μg)
total
(μg)
4d
7d
I (control group)
II (low dose)
III (medium dose)
IV (high dose)
0
5
10
30
0.00
0.25
0.50
1.50
0.00
0.35
0.70
2.10
0.00
0.60
1.20
3.60
[0181] Control group (I);
[0182] Ordinary vaccine group (II), i.e. commercially available H5-H9 subtype antigen avian influenza oil emulsi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com