Preparation method of 3,4-ethene dioxythiophene
A technology of ethylenedioxythiophene and dihydroxythiophene, which is applied in the field of synthesis of organic compounds, can solve the problems of low product yield and high cost of raw materials, and achieve the effects of high product yield, beautiful color, and no environmental pollution in the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
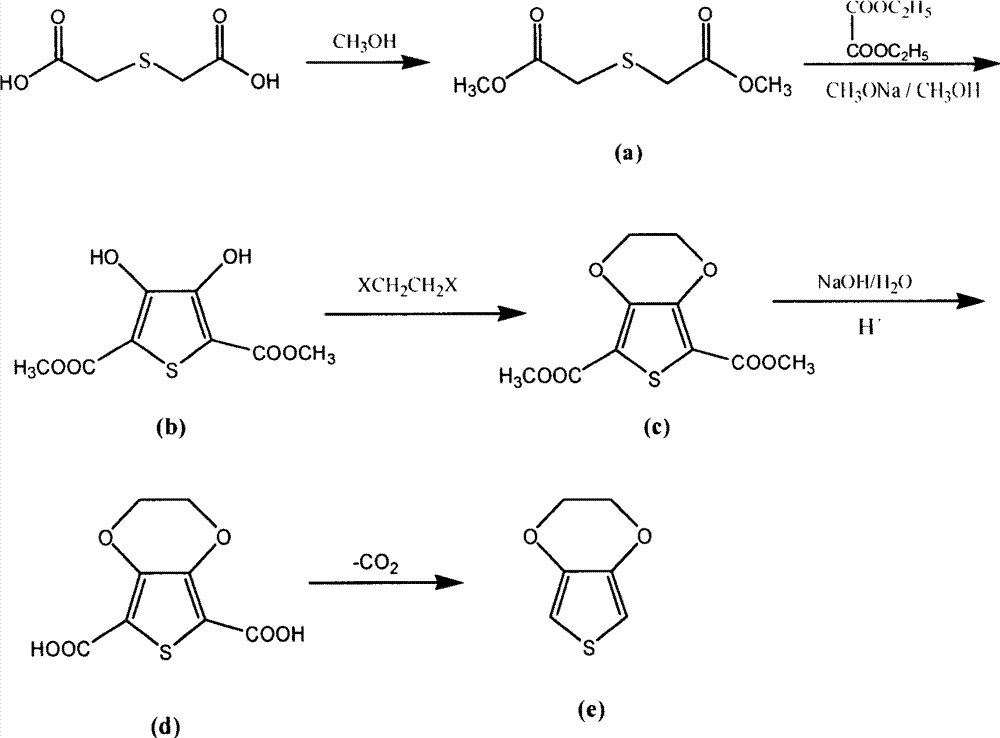
[0032] Mix 338g (2.25mol) of thiodiglycolic acid, 2560ml of methanol, and 135g of sulfuric acid (catalyst), stir, and heat up to reflux. The reaction time is 6-8h. After the reaction is completed, the methanol is distilled out under normal pressure, the temperature is lowered, and 5% lye is added. Adjust the pH to 7-7.5, extract with ether (3*300ml), and dry over anhydrous sodium sulfate. Concentrate diethyl ether under normal pressure, then distill under reduced pressure, collect the distillate with a boiling point of 172-176° C. / 1.33 kPa, and obtain 382.8 g of esterified dimethyl thiodiglycolate, which is a nearly colorless transparent liquid with a content of 99.3%. The rate is 94.8%.
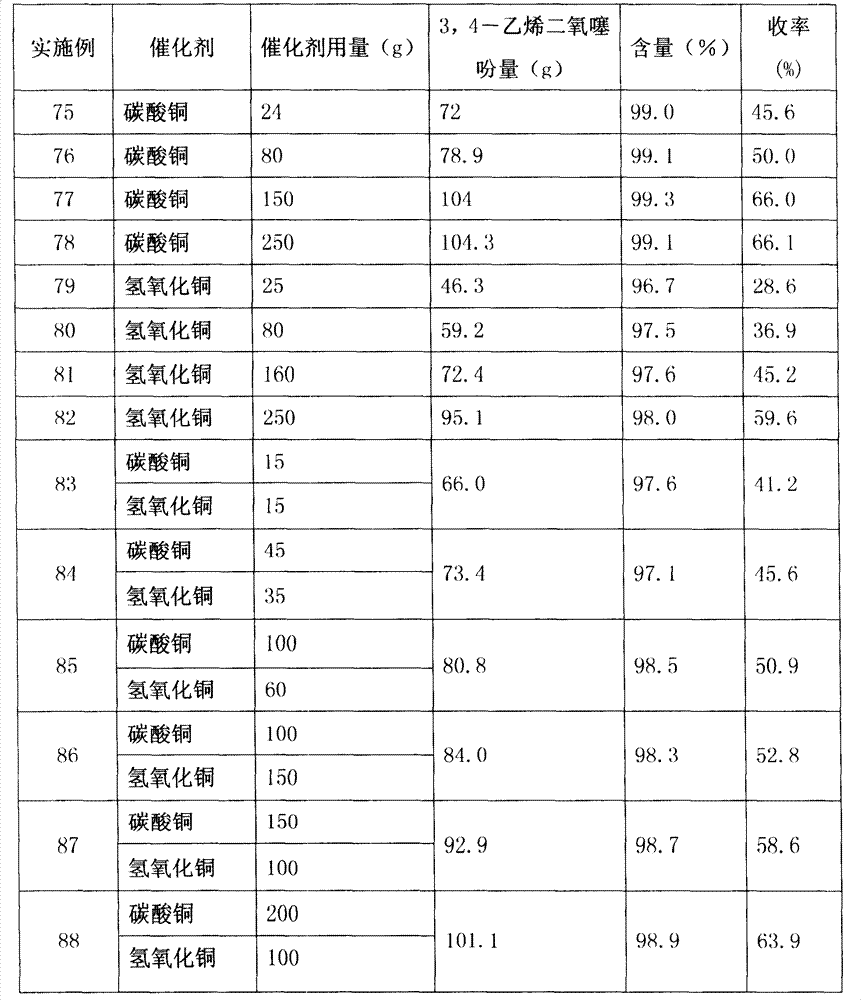
[0033] Catalyst type and consumption are changed, and other is with embodiment 1, and its result is shown in Table 1.
[0034] Table 1
[0035]
Example
catalyst catalyst
Dosage (g) Thiodiglycolic acid
Amount of dimethyl ester (g)
content(%)
Yield...
Embodiment 17
[0044] Add 380g (2.13mol) of the esterified product obtained in Example 1 into 460g (8.52mol) of sodium methoxide in 2475ml of methanol solution, add 772g (5.33mol) of diethyl oxalate dropwise under stirring, and control the temperature at 0-10°C , after the dropwise addition, the temperature was raised to reflux, and the reaction time was 3-5 hours. After the reaction was completed, methanol was recovered, 1L of water was added, acidified with hydrochloric acid, filtered and dried to obtain the condensation product 2,5-dicarboxylic acid of off-white solid powder Dimethyl ester-3,4-dihydroxythiophene 455g, content 99.4%, yield 92%.
[0045] The amount of sodium methylate was changed, and the others were the same as in Example 17. The results are shown in Table 4.
[0046] Table 4
[0047]
[0048] The amount of diethyl oxalate was changed, and others were the same as in Example 17. The results are shown in Table 5.
[0049] table 5
[0050]
[0051] Prep...
Embodiment 26
[0053] The condensation product 440g (1.9mol), 1198g (6.38mol) 1,2-dibromoethane, 503g (3.65mol) K 2 CO 3 105g of cetyltrimethylammonium bromide (phase transfer catalyst) was added in 4500ml of toluene (solvent), stirred and heated up, the temperature was controlled at 90°C, and the reaction time was 10-15h. After the reaction was completed, the material was concentrated under reduced pressure to recover the solvent and the After cooling the reacted 1,2-dibromoethane, add 5L of water, stir at room temperature for 30 minutes, and then carry out suction filtration, water washing and drying in sequence to obtain the etherification product 2,5-diformic acid methyl ester of off-white to gray solid powder. 3,4-ethylenedioxythiophene 319g, content 98.5%, yield 64%.
[0054]The amount of 1,2-dibromoethane is changed, and others are the same as in Example 26. The results are shown in Table 6.
[0055] Table 6
[0056]
[0057] Will K 2 CO 3 Consumption changes, and other...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com