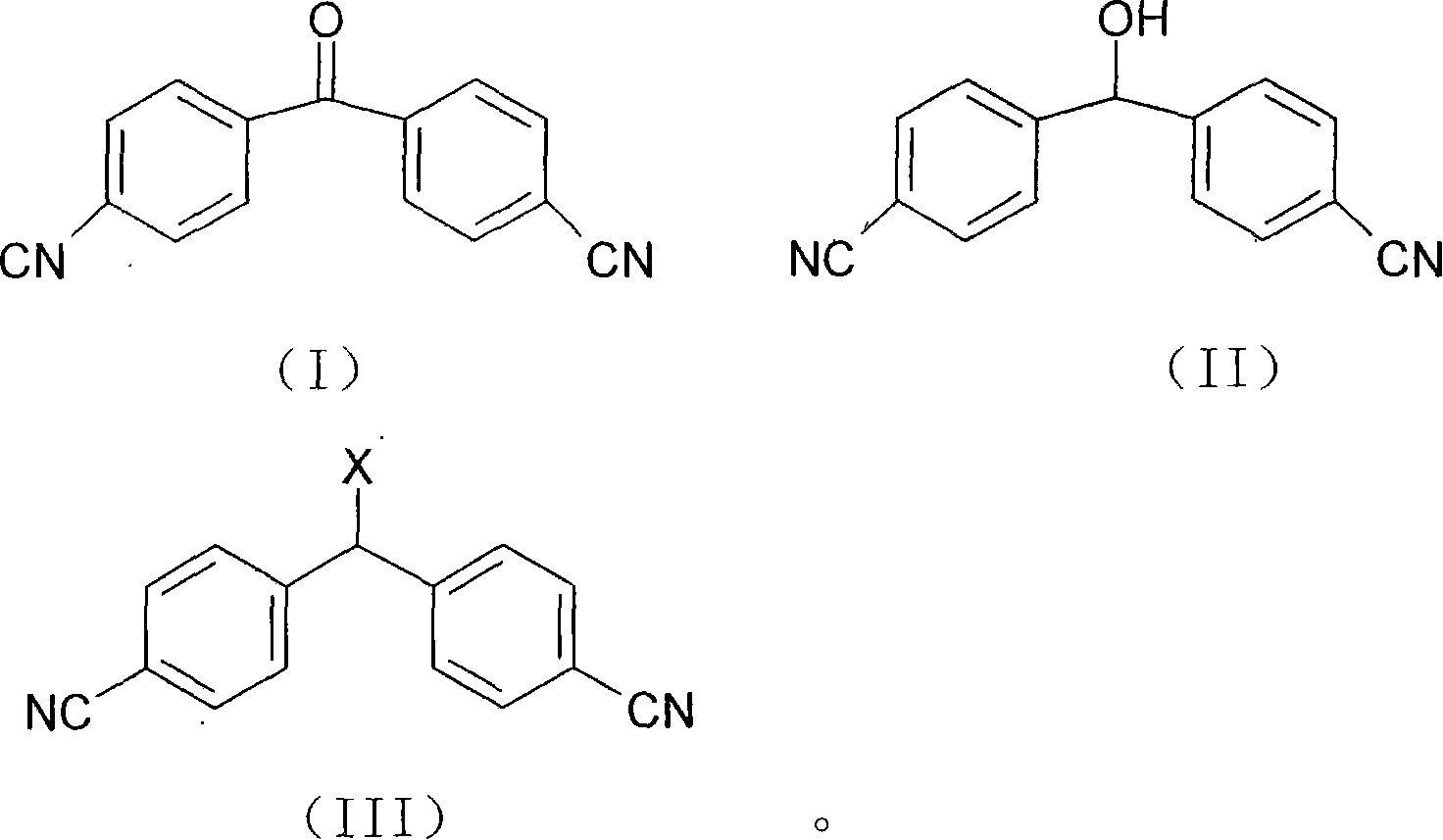
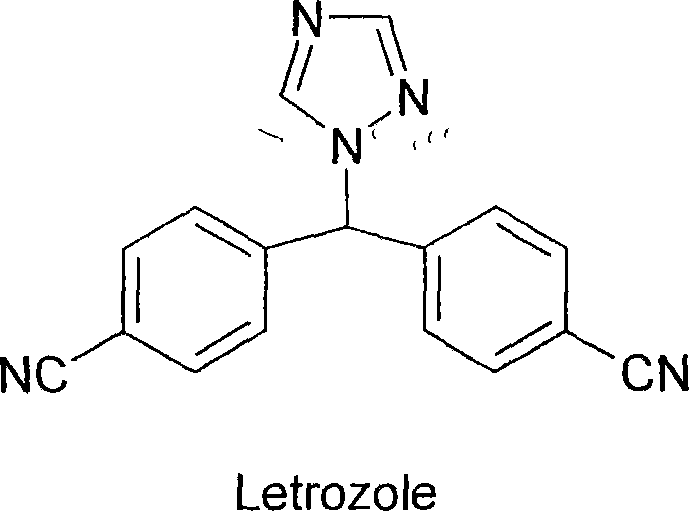
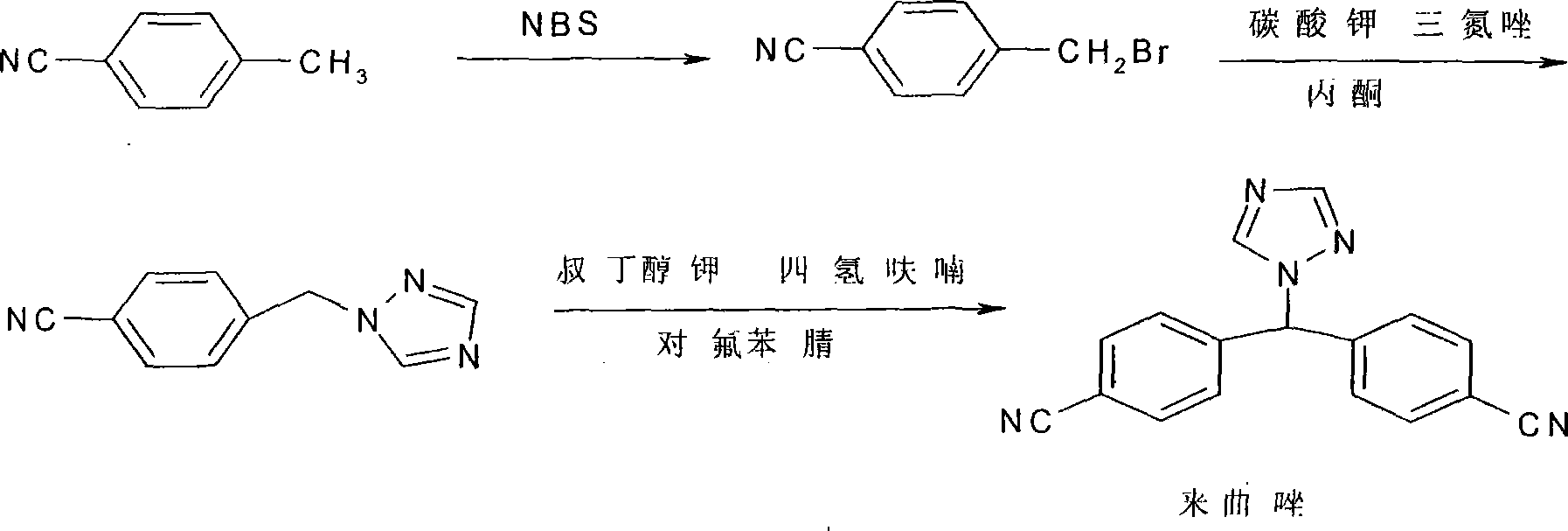
Prepn process of letrozole
A technology for letrozole and a compound is applied in the field of preparation of aromatase inhibitors, can solve problems such as unfavorable industrial production, high toxicity of cyanide, hidden danger of production safety, etc., and achieves the effects of reaction safety, yield improvement, and three-waste reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 4, the preparation of 4'-dicyanobenzhydryl alcohol
[0031] Cool the mixture of 1000ml methanol and 100g 4,4'-dicyanobenzophenone with ice water below -5°C, add 15.2g NaBH in batches 4 , ensure that the reaction temperature in the whole process is below 5°C, after the addition is complete, keep the temperature of the mixture at about 5°C and stir for 1 hr to complete. The reaction system was neutralized to neutral with glacial acetic acid, then concentrated under vacuum at 45°C; the concentrate was dissolved in 300ml CH 2 Cl 2 and 250 water for layering, then the organic phase was separated, washed with saturated brine until the water layer was free of impurities and the solvent was transparent, and was washed with anhydrous Na 2 SO 4 Drying, decolorization, and concentration yielded 93 g of white crystals, yield: 93%, melting point: 156°C~159°C.
Embodiment 2
[0032] Example 2 Preparation of 4,4'-dicyanodiphenylchlorosane
[0033] Weigh 150g of 4,4'-dicyanobenzhydrin, 1500ml of chloroform and 6g of pyridine, add them into a three-necked flask equipped with a thermometer and a condenser tube, heat, and when a slow reflux occurs, start to drop 153g of chlorinated chlorinated Sulfone, the solution slowly turned golden yellow, and the dropwise addition was completed in about 50 minutes. Then medium-speed reflux and start timing, react 4.5hr, complete. Heating was stopped and it turned yellow. Continue to stir and cool to room temperature, then cool with ice water, slowly add water to make the thionyl chloride react and generate smoke, until no gas is produced, adjust the pH to neutral with 5% sodium hydroxide solution. Transfer to a separatory funnel to stand still, separate layers, extract the aqueous layer with chloroform again 1 to 2 times, combine the organic layers, wash 3 to 4 times with water, wash 2 times with saturated saline...
Embodiment 3
[0034] The preparation of embodiment 3 letrozole
[0035] In a 2000ml three-necked flask, add 54gl, 2,4-triazole, 80gK 2 CO 3 and 1500ml of CHCl 3 , stirred for 30 minutes, heated, refluxed, stirred vigorously, and added dropwise 50g of 4,4'-dicyanodiphenyl chloride and 100ml of CHCl within 30 to 40 minutes 3 solution, complete, keep vigorously stirring and reflux reaction for 31hr, and the reaction is complete. Cool the reaction solution to normal temperature, filter out the solid matter, wash with chloroform for 1-2 times, wash the filtrate with glacial acetic acid aqueous solution to neutrality, wash with water for 2-3 times, wash with saturated saline for 2-3 times, and dry with anhydrous sodium sulfate , concentrated to dryness to obtain 50 g of letrozole crude product.
[0036] The crude product was recrystallized with a mixed solvent of chloroform and ethanol, and decolorized to obtain 41 g of the product with a yield of 67%. Melting point: 183°C-185°C, purity 99.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com