1,4-bis(4-amino-benzene oxymethylene) cyclohexyl and preparation and application thereof
A technology of aminophenoxymethylene and nitrophenoxymethylene, applied in 1 field, can solve problems such as unfavorable polymerization reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
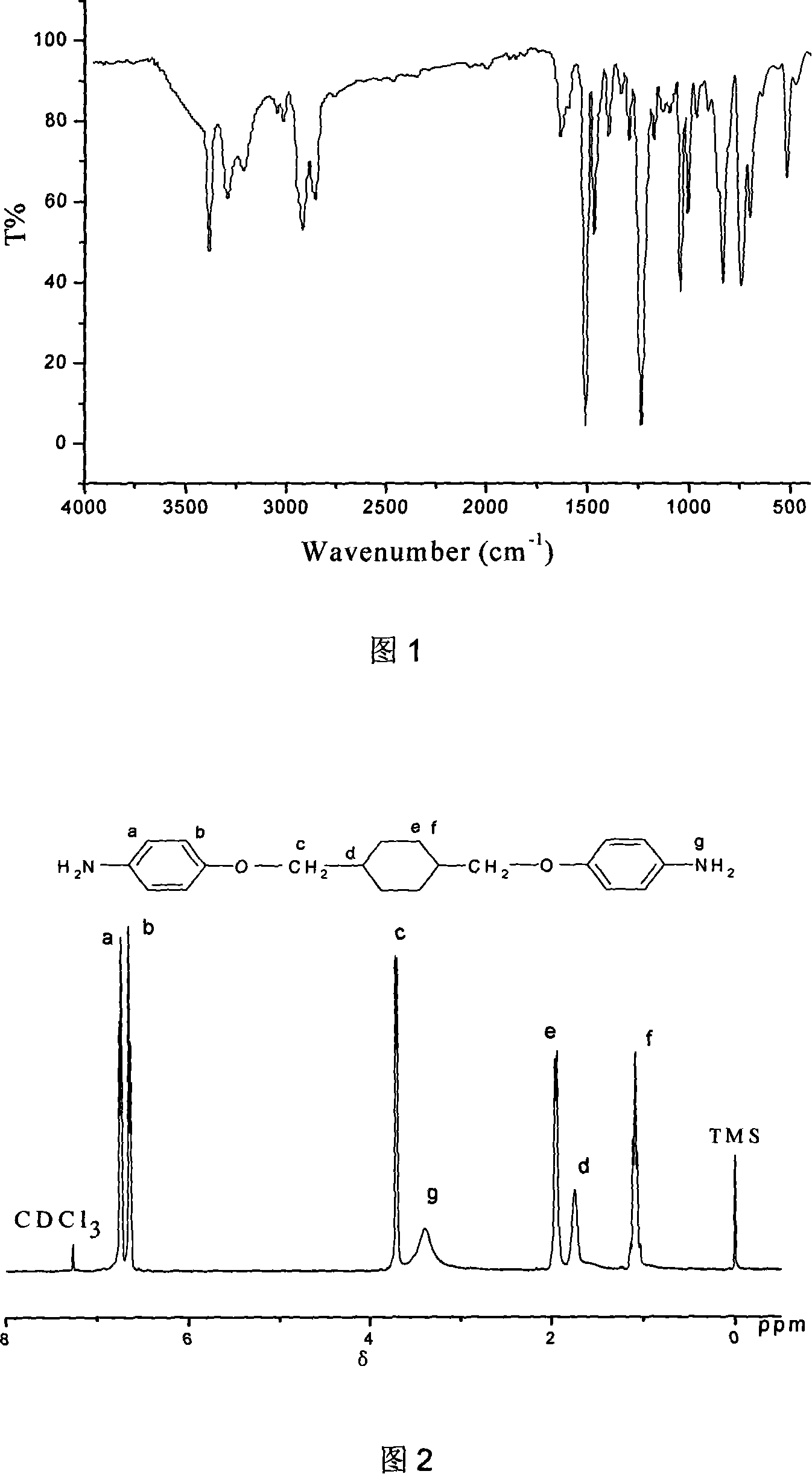
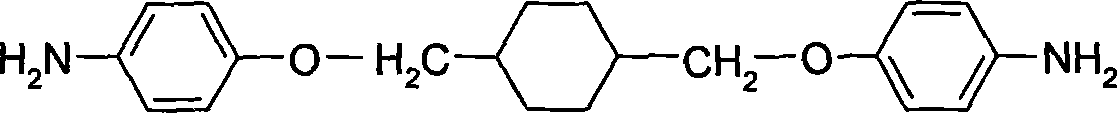
Image
Examples
Embodiment 1
[0022] (a) In a 150mL three-neck flask equipped with a stirrer, a reflux condenser, a thermometer, and a nitrogen conduit, add 1.5 grams (60%) of sodium hydride, and add 1,4-cyclohexane dropwise under ice-water bath cooling and nitrogen protection. Alkanedimethanol solution (2.0 g of 1,4-cyclohexanedimethanol dissolved in 20 mL of N,N-dimethylformamide), kept slightly boiling. Stir for 2 hours after dropping, then slowly add p-chloronitrobenzene solution (4.8 grams of p-chloronitrobenzene is dissolved in 30mL N, N-dimethylformamide), then rise to room temperature and continue to stir for 20 hours. The reaction solution was poured into 400 mL of water, filtered, and dried to obtain 4.5 g of yellow solid powder: 1,4-bis(4-nitrophenoxymethylene)cyclohexane, with a yield of 84%. The product was heated to 100°C in N,N-dimethylformamide to dissolve, filtered while hot, and the filtrate was cooled to precipitate light yellow crystals, dried under vacuum at 80°C for 12 hours to obtain...
Embodiment 2
[0025] (a) In a 150mL three-neck flask equipped with a stirrer, a reflux condenser, a thermometer, and a nitrogen conduit, add 1.5 grams (60%) of sodium hydride, and add 1,4-cyclohexane dropwise under ice-water bath cooling and nitrogen protection. Alkanedimethanol solution (2.0 g of 1,4-cyclohexanedimethanol dissolved in 20 mL of N,N-dimethylformamide), kept slightly boiling. Stir for 2 hours after dropping, then slowly add p-fluoronitrobenzene solution (4.3 grams of p-fluoronitrobenzene is dissolved in 30mL N, N-dimethylformamide) dropwise, then rise to room temperature and continue stirring for 15 hours. The reaction solution was poured into 400 mL of water, filtered, and dried to obtain 4.0 g of yellow solid powder: 1,4-bis(4-nitrophenoxymethylene)cyclohexane, with a yield of 75%. After the product was heated to 100°C in N,N-dimethylformamide to dissolve, it was filtered while it was hot, and the filtrate was cooled to precipitate light yellow crystals, which were dried un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com