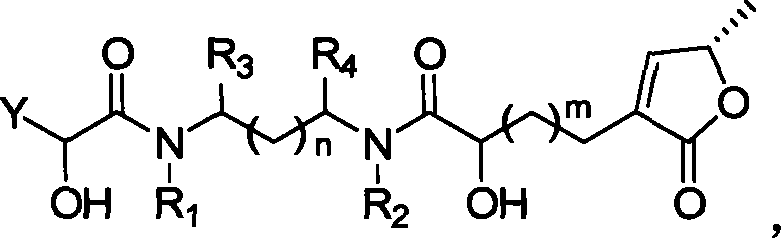
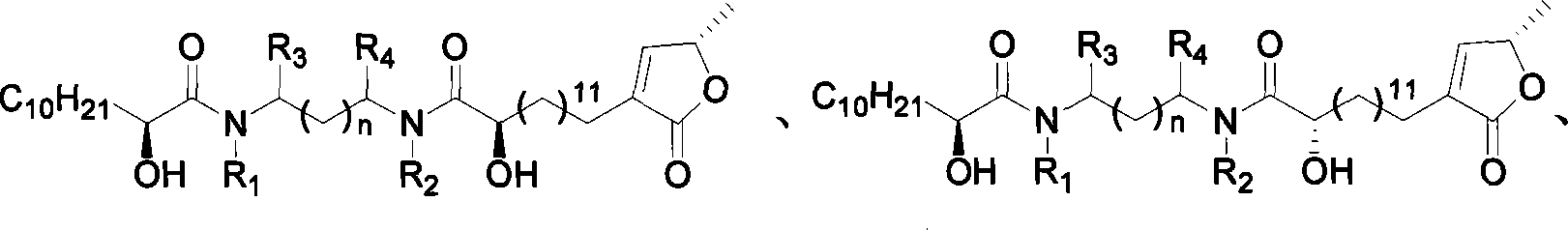
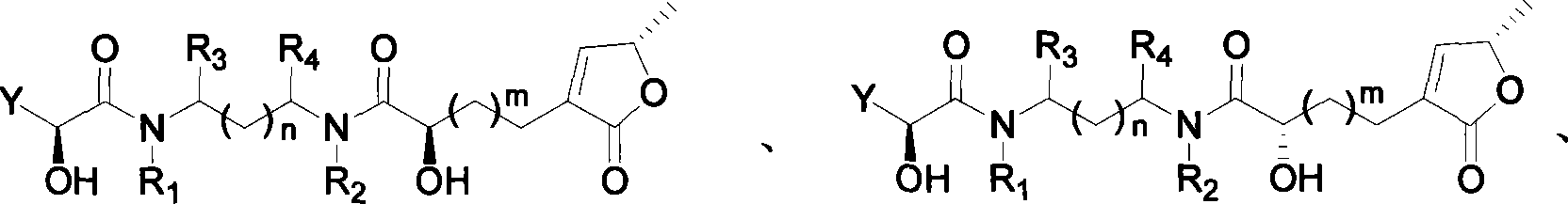Lactone compound of sweetsop connected through amido bond, synthetic method and application
A technology of anemone lactone and compound, which is applied in the field of synthesis of chiral anemone lactone analogues, and can solve problems such as low synthesis efficiency, poor water solubility, and limited research progress of finished medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]
[0057] Under cooling in an ice-water bath, 4.14 g of Dess-Martin oxidant was added to a solution of 2.00 g of compound 18 in 50 mL of dichloromethane. The reaction system was stirred at room temperature for 2 hours, quenched with saturated sodium sulfite solution, extracted with ethyl acetate, and the combined organic phase was washed with saturated sodium bicarbonate and saturated sodium chloride in sequence, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product. The above crude product and 9.6 mL of 2-methyl-2-butene were dissolved in 40 mL of tert-butanol, cooled to 0°C, and a 10 mL aqueous solution of 2.22 g of sodium chlorite and 3.35 g of potassium dihydrogen phosphate was slowly added dropwise. The reaction mixture was stirred overnight at room temperature, the solvent was spinned out under reduced pressure, extracted with ethyl acetate, the organic phases were combined and washed with saturated brine, dried over anhydrous ...
Embodiment 2
[0064]
[0065] Under cooling in an ice bath, a solution of 102 mg TBDPSCl in 5 mL of dichloromethane was slowly added dropwise to 100 mg of diol 20 and 50 mg of imidazole in 5 mL of dichloromethane. After the reaction mixture was stirred at room temperature for 2 hours, 0.58 mL of Li-Pr 2 After NEt was cooled to 0°C, 0.12 MLMOMCl was slowly injected. The reaction system was stirred overnight at room temperature, quenched with saturated ammonium chloride, and extracted with ethyl acetate. The combined organic phases were washed with 1N hydrochloric acid saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatography gave a colorless Liquid 175mg, yield 90%.
[0066] [α] D 25 =-15.30 (c1.70, CHCl 3 );
[0067] 1 H NMR (400MHz, CDCl 3 ): 1.05(9H, s), 1.29(20H, m), 1.62(4H, m), 2.30(2H, t, J=7.5Hz), 3.34(3H, s), 3.64(6H, m), 4.64 (1H, d, J=6.7 Hz), 4.77 (1H, d, J=6.8 Hz), 7.39 (6H, m), 7.68 (4H, m) ppm;
[0068] MS (ESI, m / z): 6...
Embodiment 3
[0071]
[0072] At -78°C, slowly add 0.34 mL (1.6 Minhexane) n-butyl lithium dropwise to 0.12 mL of diisopropylamine in 3 mL of THF, and after stirring for 30 minutes, slowly add 0.16 g of compound 21 in 5 mL of anhydrous THF dropwise to the reaction system solution, and after continuing to stir for 30 min, a freshly prepared solution of 0.087 g of aldehyde 23 in 3 mL of anhydrous THF was slowly added dropwise. After the reaction system was stirred for 2 hours, it was quenched with saturated ammonium chloride, and the system was extracted with ethyl acetate. The combined organic phases were washed with saturated sodium chloride, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product that was dissolved in 10 mL of THF and added with 1 mL of 10 %H 2 SO 4 , stirred at room temperature for 24 hours, quenched with saturated sodium bicarbonate and extracted with ethyl acetate, the combined organic phases were washed with saturated sodium chlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com