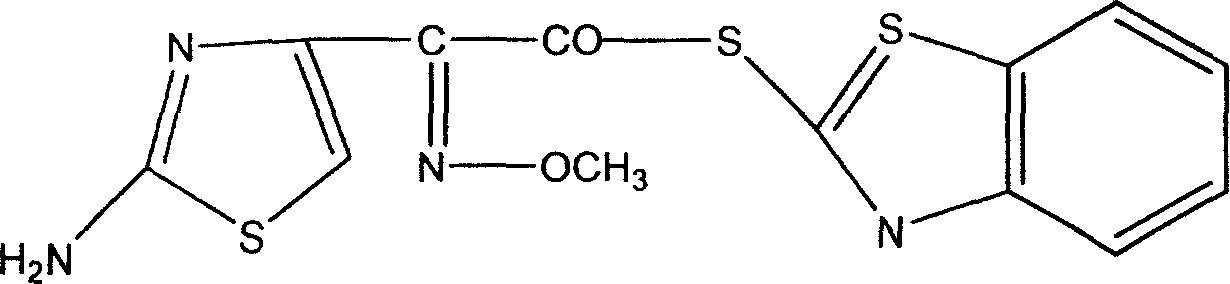
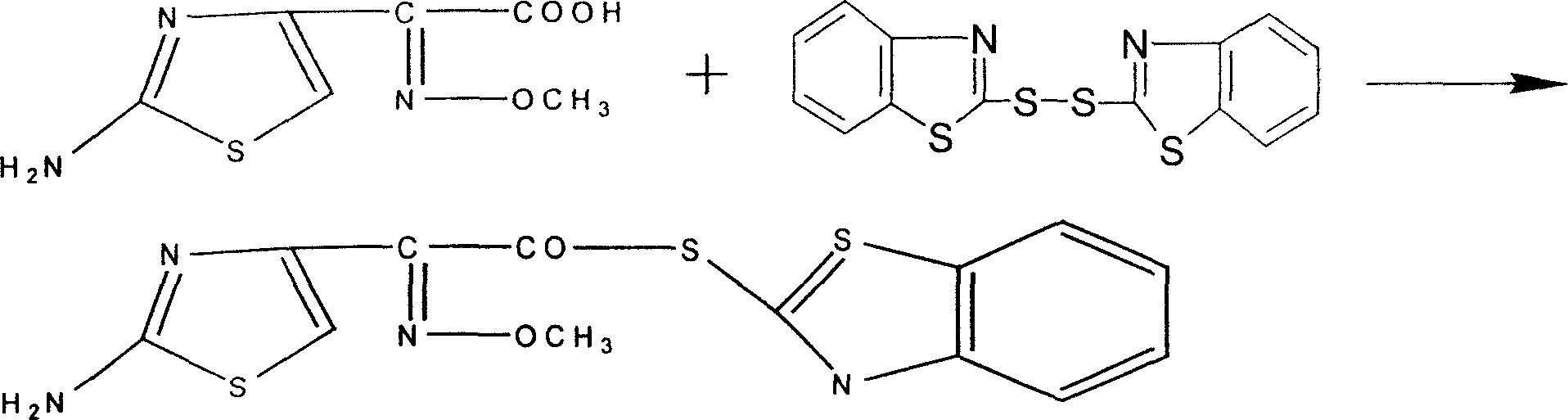
New technique for catalytic synthesis of AE active ester
A new technology, active ester technology, applied in the production process field of key intermediate - AE-active ester, can solve the problems of mild production and operation conditions, achieve the effect of mild production and operation conditions, reduce operation intensity, and reduce operation difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Raw material ratio: 200 kg of aminothioxamic acid
[0022] Dibenzothiazole disulfide 250 kg
[0023] Pyridine 1.5 kg
[0024] Mixed solvent 1600 liters
[0025] Wherein the volume ratio of mixed solvent acetonitrile and dichloromethane is acetonitrile: dichloromethane=0.9:1
[0026] Triethylamine 80 kg
[0027] Phosphite triethyl ester 160 kg
[0028] 200 liters of mixed solvent in the rinse mother liquor
[0029] New acetonitrile 100 liters
[0030] New dichloromethane 200 liters
[0031] (1) Preparation of mixed solvent
[0032] Add 1,100 liters of new dichloromethane and 1,000 liters of new acetonitrile into a 3,000-liter well-sealed reactor, add 120 kg of phosphorus pentoxide under nitrogen protection, seal the reactor, heat up and distill, and collect at 80-110°C The distillate is placed in a dry and airtight storage tank for later use.
[0033] (2) Reaction
[0034] In the reaction kettle, first add 1600 liters of distilled mixed solvent, then add aminot...
Embodiment 2
[0038] Embodiment 2: according to the technique of embodiment 1, the proportioning of different raw materials is:
[0039] Aminothioxamic acid 200kg
[0040] Dibenzothiazole disulfide 250 kg
[0041] Pyridine 1 kg
[0042] Mixed solvent 1500 liters
[0043] Wherein the volume ratio of mixed solvent acetonitrile and dichloromethane is acetonitrile: dichloromethane=1.0:1
[0044] Triethylamine 82 kg
[0045] Phosphite triethyl ester 162 kg
[0046] The product yield is 91%.
Embodiment 3
[0047] Embodiment 3: according to the technique of embodiment 1, the proportioning of different raw materials is:
[0048] Aminothioxamic acid 200kg
[0049] Dibenzothiazole disulfide 255 kg
[0050] Pyridine 2 kg
[0051] Mixed solvent 1700 liters
[0052] Wherein the volume ratio of mixed solvent acetonitrile and dichloromethane is acetonitrile: dichloromethane=1.0:1
[0053] Triethylamine 80 kg
[0054] Phosphite triethyl ester 158 kg
[0055] The product yield is 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com