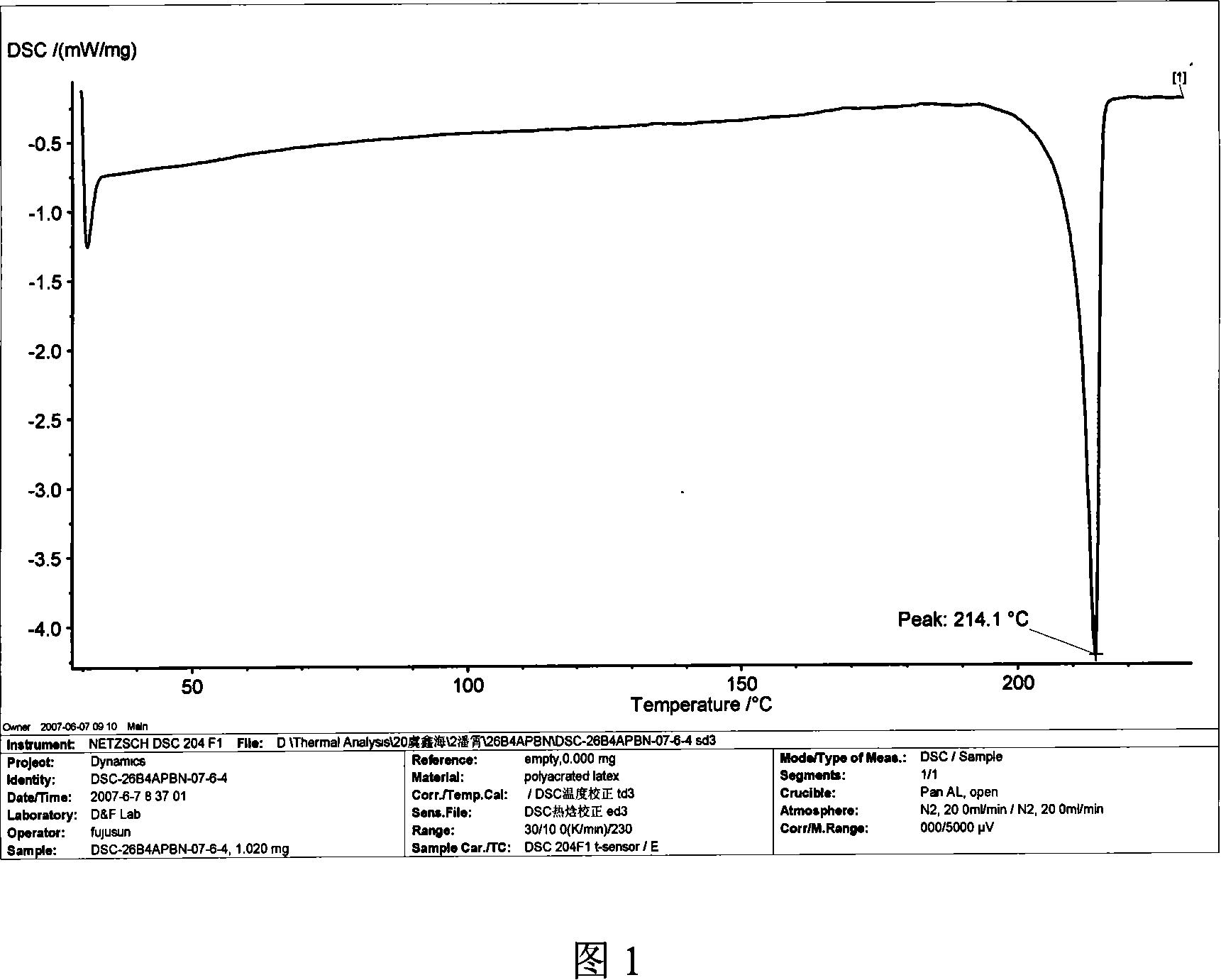
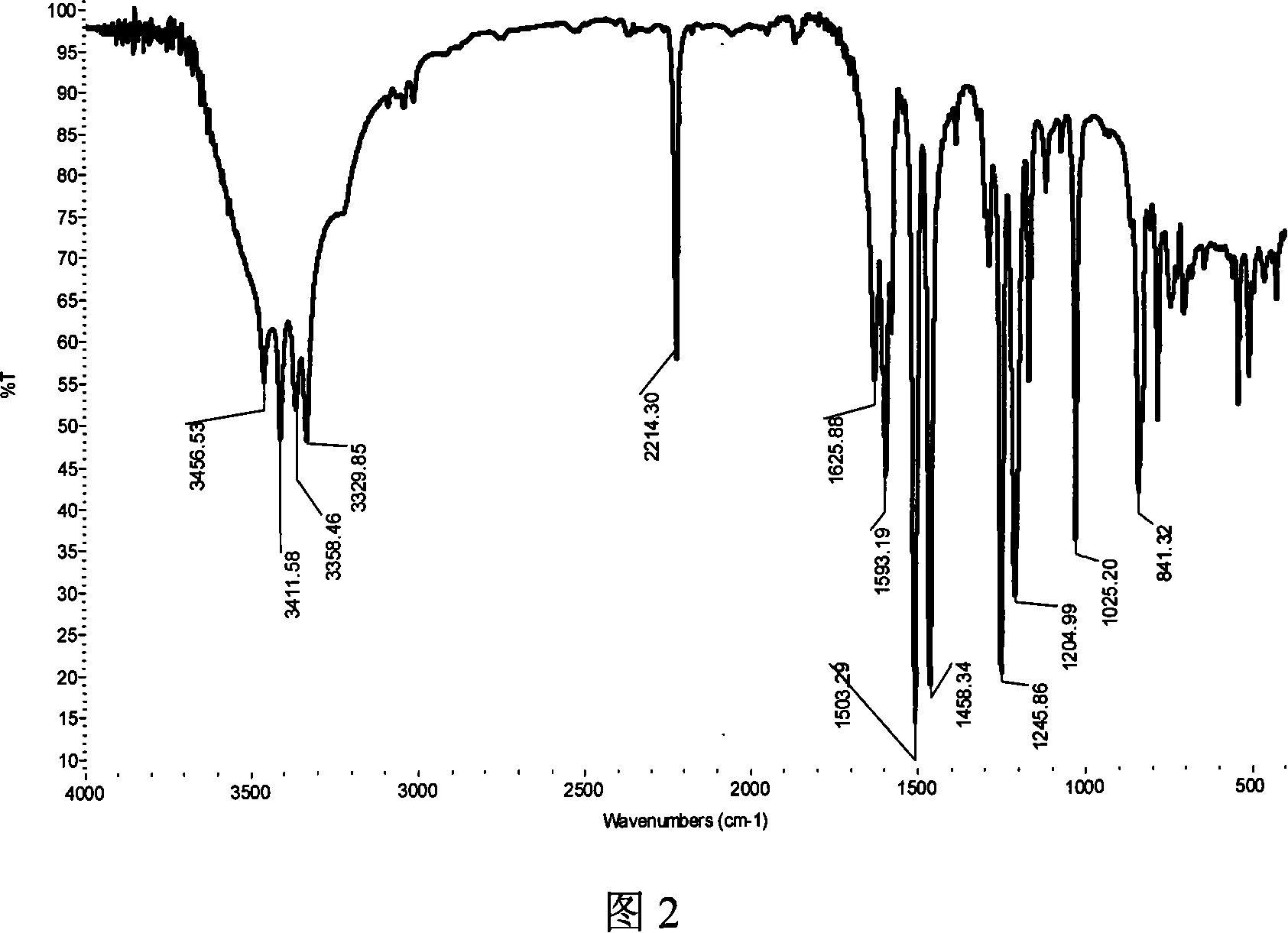
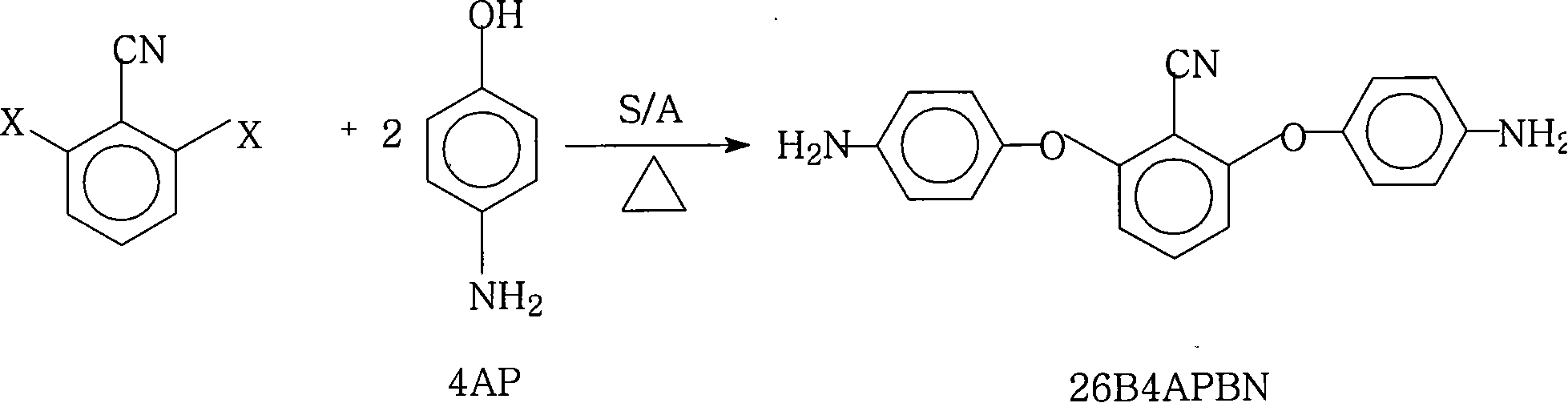
Method for preparing 2,6-di(4-amino-benzene oxygen) cyanobenzene
A technology of aminophenoxy and benzonitrile, which is applied in two fields, can solve the problems of unsatisfactory industrial production, target product yield and low melting point, and achieve the effects of low production cost, simple and feasible preparation method, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 21.8 grams (0.2 moles) of 4-aminophenol (4AP), 27.6 grams (0.2 moles) of potassium carbonate, 200 milliliters of N-methyl-2-pyrrolidone (NMP) and 200 milliliters of xylene into the reaction flask, blow nitrogen After 2 hours of reflux and water separation reaction, cool the reaction solution to 80 ° C, add 17.2 grams (0.1 mole) of 2,6-dichlorobenzonitrile (26DCBN), heat and reflux reaction, and separate part to all xylene , to increase the reaction temperature, react at a temperature range of 170°C to 200°C for 12 hours, cool the reaction solution to 80°C, filter while it is hot, remove inorganic salts, keep the mother liquor, cool to room temperature, add water dropwise, precipitate the product, filter (collect the mother liquor to reclaim the organic solvent), wash the filter cake, and dry in vacuo to obtain 28.9 grams of brown 2,6-bis(4-aminophenoxy)benzonitrile (26B4APBN) solid product, according to the theoretical yield (31.7 grams ) and the actual amount of ga...
Embodiment 2
[0039] Add 21.8 grams (0.2 moles) of 4-aminophenol (4AP), 2.76 grams (0.02 moles) of potassium carbonate, and 90 milliliters of anhydrous N-methyl-2-pyrrolidone (NMP) into the reaction flask, argon, and reflux reaction After 0.5 hours, cool the reaction solution to 80°C, add 26.1 grams (0.1 moles) of 2,6-dibromobenzonitrile (26DBBN), heat up, and react at a temperature range of 170°C to 200°C for 3 hours, then cool the reaction liquid to 80°C, filter while hot, remove inorganic salts, retain the mother liquor, cool to room temperature, add water dropwise, precipitate the product, filter (collect the mother liquor to recover the organic solvent), wash the filter cake, and vacuum dry to obtain 12.1 grams of brown The solid product of 2,6-bis(4-aminophenoxy)benzonitrile (26B4APBN) was calculated to have a yield of 38.2% based on the theoretical yield (31.7 g) and the actual amount obtained.
Embodiment 3
[0041] 24.0 grams (0.22 moles) of 4-aminophenol (4AP), 26.5 grams (0.25 moles) of sodium carbonate, 200 milliliters of anhydrous N-methyl-2-pyrrolidone (NMP) and 40 milliliters of anhydrous N, N-di Methyl acetamide (DMAc) was added to the reaction flask, nitrogen was passed, and after 5 hours of reflux reaction, the reaction solution was cooled to 80°C, 13.9 grams (0.1 moles) of 2,6-difluorobenzonitrile (26DFBN) was added, and heated Raise the temperature, react in the temperature range of 170°C to 200°C for 7 hours, cool the reaction solution to 80°C, filter while it is hot, remove the inorganic salt, keep the mother liquor, cool to room temperature, add water dropwise, precipitate the product, filter (collect the mother liquor, to reclaim the organic solvent), wash the filter cake, and dry in vacuo to obtain 25.9 grams of brown 2,6-bis(4-aminophenoxy)benzonitrile (26B4APBN) solid product, according to the theoretical yield (31.7 grams) and the actual obtained amount, the cal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com