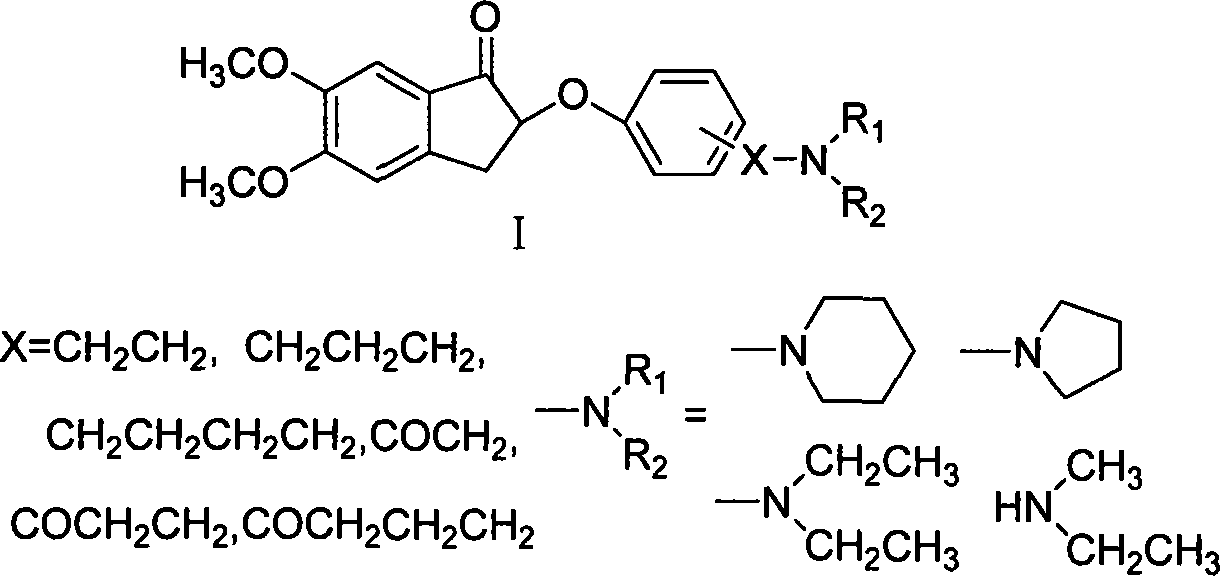
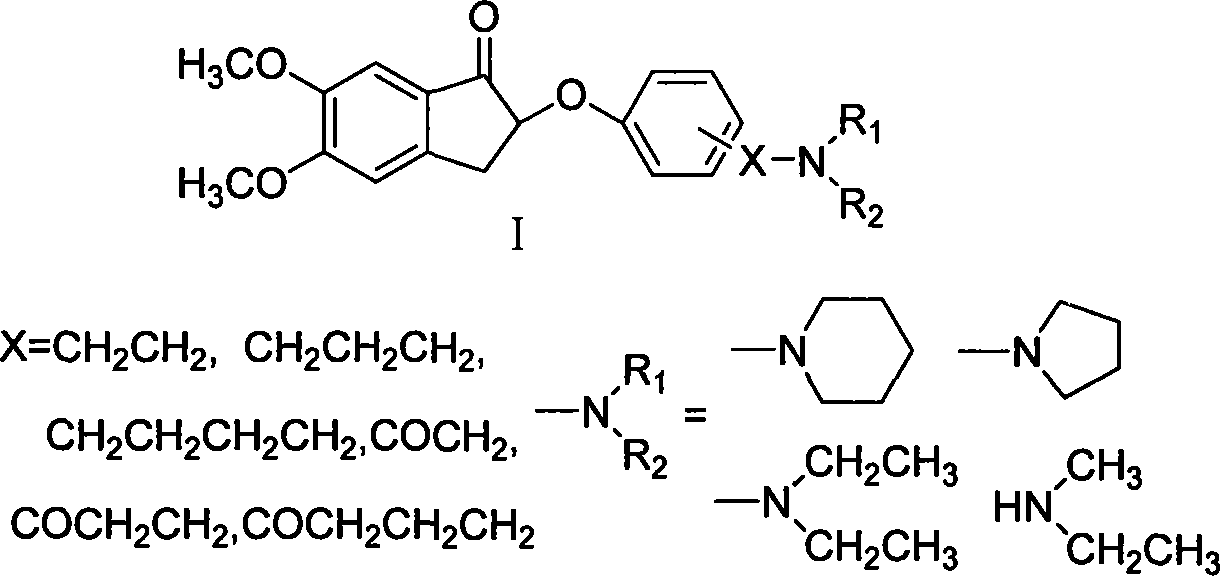
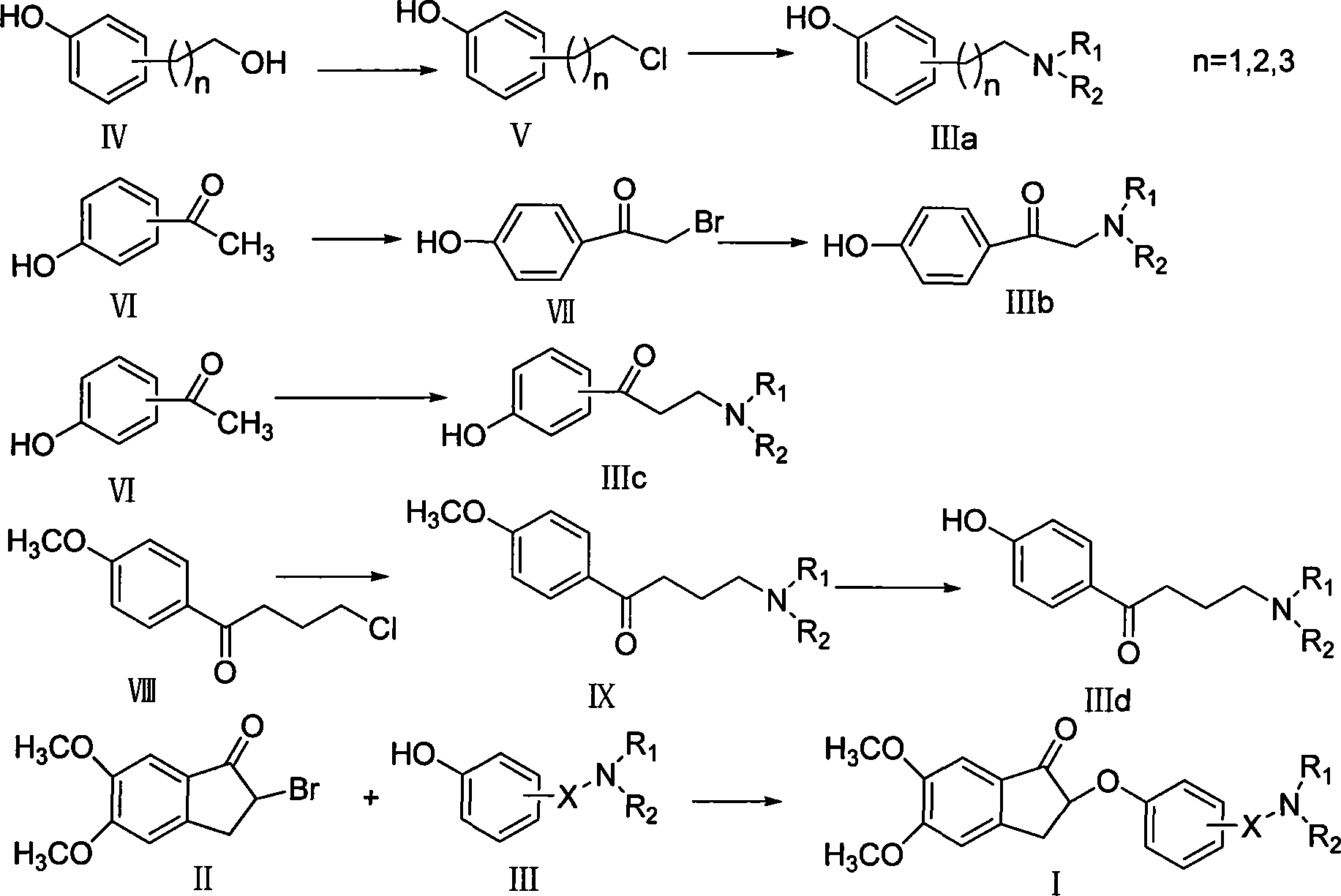
Phenoxy indanone derivatives containing alkylamino side-chain, preparation method and use thereof
A phenoxyindanone and alkylamine-containing technology, applied in the field of compound synthesis, can solve the problems of short action time, low bioavailability, poor central selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 4-[2-(pyrrolidin-1-yl)ethyl]phenol
[0032] Dissolve 0.157g (0.001mol) of 4-(2-chloroethyl)phenol in 2ml of acetonitrile, add 0.41ml (0.003mol) of triethylamine, and add 0.35ml (0.0042mol) of tetrahydropyrrole dropwise in an ice bath. Warm up to reflux and react for 10h. The solvent was evaporated to dryness under reduced pressure, 2ml of 2N hydrochloric acid and 2ml of ethyl acetate were added to the residue, and the organic layer was separated and discarded. The aqueous layer was adjusted to pH 10 with concentrated ammonia, extracted with ethyl acetate (2ml×3), the organic layers were combined, washed with saturated NaCl, anhydrous NaCl 2 SO 4 After drying, the solvent was recovered, and silica gel column chromatography (petroleum ether: ethyl acetate = 1:1, 1% triethylamine) was used to obtain 0.139 g of an oily substance with a yield of 72.4%. 1H-NMR (δ, CDCl 3 ): 6.99-7.01(d, 2H, J=8.4Hz), 6.63-6.65(d, 2H, J=8.4Hz), 2.73-2.78(m, 4H), 2.65(m, 4H), 1.82...
Embodiment 2
[0056] Example 2 1-(4-hydroxyphenyl)-2-(pyrrolidin-1-yl)ethanone
[0057] Dissolve 0.108g (0.5mmol) of α-bromo-p-hydroxyacetophenone in 4ml of anhydrous acetonitrile, add 0.21g (2.5mmol) of K 2 CO 3 , add 0.205ml (0.0025mol) tetrahydropyrrole dropwise in an ice bath, keep the temperature at 0-10°C, and react for 0.5h. After filtration, the filtrate was concentrated to dryness under reduced pressure and subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 1:2, 1% triethylamine and 1% methanol) to obtain 90 mg of a yellow powdery solid with a yield of 87.3%. 1H-NMR (δ, CH 3 OD): 7.85-7.87(d, 2H, J=8.8Hz), 6.80-6.82(d, 2H, J=8.8Hz), 4.94(s, 2H), 2.80(m, 4H), 1.87(m, 4H ).
[0058] The following compounds can be prepared in the same way:
[0059] 1-(4-Hydroxyphenyl)-2-(piperidin-1-yl)ethanone
[0060] The operation process is the same as in Example 2, except that tetrahydropyrrole is replaced by piperidine, and the yield is 84%.
[0061] 1-(4-hydr...
Embodiment 3
[0065]Example 3 1-(4-hydroxyphenyl)-3-pyrrolidinyl-1-propanone
[0066] Put 0.63g (0.0046mol) of p-hydroxyacetophenone, 0.504g (0.0168mol) of paraformaldehyde, 1.26ml (0.015mol) of tetrahydropyrrole, and 4.2ml of absolute ethanol into the reaction flask, and add 0.21ml of it dropwise under ice cooling Concentrated hydrochloric acid, heated to reflux, reacted for 10h. The solvent was distilled off under reduced pressure, 5 ml of 2N hydrochloric acid and 5 ml of ethyl acetate were added to the residue, and the organic layer was separated and discarded. The pH of the aqueous layer was adjusted to 10 with concentrated ammonia water, a large amount of precipitates formed, filtered, washed, and the filter cake was dried to obtain 0.324 g of off-white powder with a yield of 32.3%. Melting point: 148°C. 1H-NMR (6, CDCl 3 ): 7.83-7.85(d, 2H, J=8.8Hz), 6.82-6.84(d, 2H, J=8.8Hz), 3.04-3.08(t, 2H, J=7.6Hz), 2.70-2.73(t, 2H, J=7.6Hz), 2.41-2.44(m, 4H), 1.63-1.66(m, 4H).
[0067] The f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com