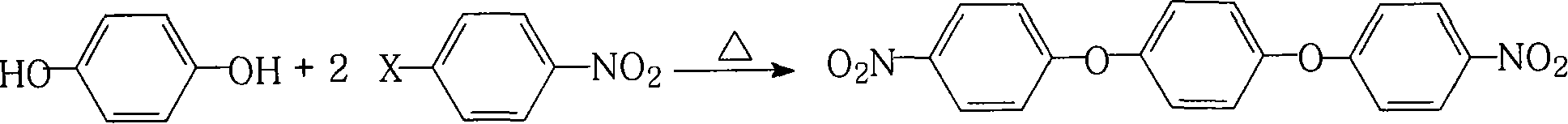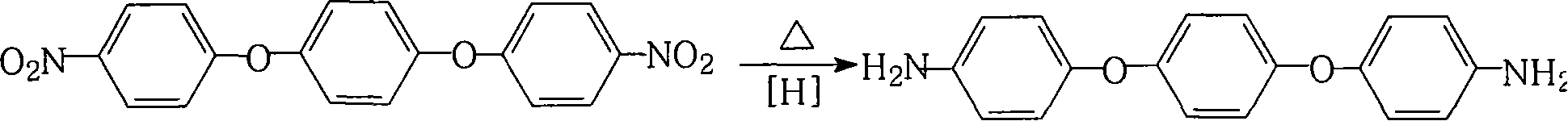Method for preparing 1,4-di(4-amino-benzene oxygen) benzene
A technology of aminophenoxy and nitrophenoxy, applied in the first field, can solve the problems of unfavorable environmental protection, complex operation process, low total yield, etc., and achieve low production cost, high yield and purity, and convenient source Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]11.0 grams (0.10 moles) of hydroquinone, 44.1 grams (0.28 moles) of 4-chloronitrobenzene, 110.4 grams (0.80 moles) of potassium carbonate, 700 milliliters of N, N-dimethylformamide and 180 milliliters Put toluene in the reaction kettle, stir, heat and reflux for water separation for 18 hours, concentrate the reaction solution, recover the solvent for recycling, cool the reactant system, add water, precipitate a yellow-green solid product, and dry to obtain 35.1 grams of 1,4- Bis(4-nitrophenoxy)benzene has a melting point of 239.6°C and a purity of 99.9%. According to the actual amount of 1,4-bis(4-nitrophenoxy)benzene obtained and the theoretical amount (35.2 grams), The calculated yield of 1,4-bis(4-nitrophenoxy)benzene was 99.7%.
Embodiment 2
[0053] 11.0 grams (0.10 moles) of hydroquinone, 44.5 grams (0.22 moles) of 4-bromonitrobenzene, 55.2 grams (0.40 moles) of potassium carbonate, 150 milliliters of N, N-dimethylacetamide and 15 milliliters of di Put toluene in the reaction kettle, stir, heat and reflux for water separation for 2 hours, concentrate the reaction solution, recover the solvent for recycling, cool the reactant system, add water, precipitate a yellow-green solid product, and dry to obtain 33.6 grams of 1,4- Bis(4-nitrophenoxy)benzene has a melting point of 239.1°C and a purity of 99.9%. According to the actual amount of 1,4-bis(4-nitrophenoxy)benzene and the theoretical amount (35.2 grams), the calculation The yield of 1,4-bis(4-nitrophenoxy)benzene was 95.5%.
Embodiment 3
[0055] 11.0 grams (0.10 moles) of hydroquinone, 28.4 grams (0.18 moles) of 4-chloronitrobenzene, 10.6 grams (0.10 moles) of sodium carbonate, 40 milliliters of N-methyl-2-pyrrolidone and 15 milliliters of dichloro Substituted benzene was placed in a reaction kettle, stirred, heated to reflux and separated from water for 10 hours, the reaction solution was concentrated, the solvent was recovered for recycling, the reactant system was cooled, water was added, a yellow-green solid product was precipitated, and dried to obtain 31.1 g of 1,4 - Bis(4-nitrophenoxy)benzene, melting point is 238.2°C, purity is 99.5%, according to the actual and theoretical amount of 1,4-bis(4-nitrophenoxy)benzene obtained (31.7 grams) , The calculated yield of 1,4-bis(4-nitrophenoxy)benzene was 98.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com