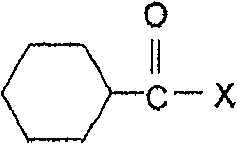
Method for preparing low sulfonated caprolactam
A technology of caprolactam and sulfonation, applied in the field of preparation of caprolactam, can solve the problems of no solution, intensified side reaction of sulfonation, uneconomical and the like, achieve high conversion rate and yield, ensure product quality, and reduce the effect of residual concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
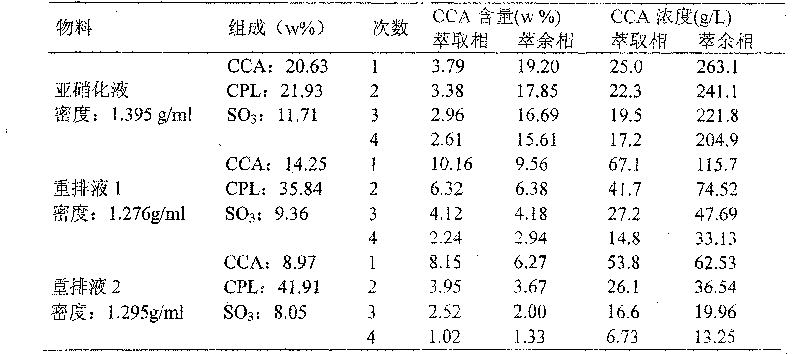
[0037]Embodiment 1: Get the nitrosation liquid of the toluene method industrial device, leave standstill and go out light phase, analyze and obtain its weight composition: 20.63% of cyclohexyl formic acid, 21.93% of caprolactam, 11.71% of sulfur trioxide. Take a certain amount of heavy phase and place it in a 250ml three-port glass reactor, add n-hexane with an equal volume to the heavy phase, stir vigorously at 60°C, and use a constant temperature water bath to control the temperature with an accuracy of ±1°C. After the material reaches the set temperature, it is allowed to stand still to balance, and samples of light and heavy phases are taken respectively, which are recorded as 1#. Separate the light and heavy phases, then add n-hexane of equal volume to the heavy ones, repeat the above steps, and record the samples as 2#, 3# and 4# in turn to achieve the effect of multi-stage extraction. The contents of cyclohexyl formic acid in the extract phase and the raffinate phase we...
Embodiment 2
[0043] Example 2: Take 115.2 g of cyclohexyl formic acid and dissolve it in the mixed hydrocarbon of cyclohexane and n-hexane, wherein 20 g of cyclohexane and 50 g of n-hexane. Add 51.5g free SO 3 Fuming sulfuric acid with a concentration of 63.5% was reacted to obtain mixed anhydrides. Add 60.1 g of nitrosyl sulfuric acid with a concentration of 81% to the mixed acid anhydride under normal pressure to carry out nitrosation reaction at a temperature of 73° C. for 15 minutes. After the reaction was completed, it was quenched and left to stand, and 200.2 g of the heavy phase product was separated. Get 100g heavy phase to analyze, obtain its composition as cyclohexyl formic acid 17.40%, caprolactam 21.30%, SO 3 11.07%, sulfuric acid 47.53%. Add 29.84g of cyclohexanone oxime into 122.56g of n-hexane to form an oxime solution. Add the oxime solution dropwise to the remaining 100.2 g of the nitrosation heavy phase product, and stir rapidly to ensure uniform heat transfer. Added...
Embodiment 3
[0045] Example 3: At 35°C, cyclohexyl formic acid and n-hexane were sent to the circulation pump at a flow rate of 207.5kg / h and 144.1kg / h for mixing, and separated by flash evaporation and decanting to obtain 65% cyclohexyl formic acid Solution, add free SO at a flow rate of 76.4kg / h 3 Fuming sulfuric acid with a concentration of 63.5% is mixed, and the temperature is controlled not to be higher than 40° C. through external circulation heat exchange. The mixed acid anhydride enters the first stage of the 9-stage fully mixed flow series reactor with a flow rate of 284.8kg / h, and the concentration is 72% nitrosyl sulfuric acid solution and n-hexane with a flow rate of 124.1kg / h and 5693.7kg / h respectively Graded addition, nitrosation reaction. The pressure is 113 kPa, the corresponding temperature is 73° C., and the residence time is 20 minutes. The reaction product is 374.9kg / h, and the analysis composition is 23.11% cyclohexyl formic acid, 16.01% caprolactam, SO 3 11.12%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com