Amalgamation protein of human glucagons-like peptide-1 and uses thereof
A technology for glucagon and glucagon tumor, which is applied in the field of recombinant protein and its therapeutic application, can solve the problems of difficult polypeptide technology, high production cost, environmental pollution, etc., and achieves short half-life, low production cost, The effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Synthetic Pro237 gene sequence
[0063] According to the sequence: Pro237 gene-stop codon TAG, chemically synthesized base sequence with a length of 513bp, as follows:
[0064] BamHI
[0065] 5'- ggactt ATGAAACGTCATAGTGAAGGGGCCTTTACCAGTGAT
[0066] Met Lys Arg His Ser Glu Gly Thr Phe Thr Ser Asp
[0067] GTGAGTTCTTACTTGGAGGGCCAGGCAGCAAAGGAATTCATT
[0068] Val Ser Ser Tyr Leu Glu Gly Gln Ala Ala Lys Glu Phe Ile
[0069] GCTTGGCTGCTGAAAGGCCGAGGAAGGCGACATGCTGATGGA
[0070] Ala Trp Leu Val Lys Gly Arg Gly Arg Arg His Ala Asp Gly
[0071] TGCTTCTCTGATGATATGAACACGATTCTCGATAACCTTGCCGCC
[0072] Ser Phe Ser Asp Glu Met Asn Thr Ile Leu Asp Asn Leu Ala Ala
[0073] AGAGACTTCATCAACTGGCTGATTCAAACCAAGATTCACTGAC
[0074] Arg Asp Phe Ile Asn Trp Leu Ile Gln Thr Lys Ile Thr Asp
[0075] AAGAAACATAGTGAAGGGGCCTTTACCAGTGATGTGAGTTCT
[0076] Lys Lys His Ser Glu Gly Thr Phe Thr Ser Asp Val Ser Ser
[0077] TACTTGGAGGGCCAGGCAGCAAAGGAATTCATTGCTTGGCTG
[0078] Tyr Leu G...
Embodiment 2
[0096] Example 2 Transform Escherichia coli with an expression vector containing the Pro237 gene sequence to obtain a genetically engineered strain
[0097] Transform the constructed recombinant plasmid pET32a-Pro237 into competent Escherichia coli BL21(DE3), keep it in an ice bath for 30 minutes, raise the temperature to 42°C in a water bath for 90 seconds, then quickly transfer it to an ice bath, cool for 2-3 minutes, and transfer to the transformation mixture Add 500 μl of LB culture solution to the medium, mix well, and shake culture at 37°C ( image 3 )
[0098] image 3 Agarose gel electrophoresis results for recombinant plasmid pET32a-Pro237 digested and identified. In the figure, 1 is the pET32a empty vector control, 2 is the recombinant plasmid pET32a-Pro237, 3 is the result of pET32a-Pro237 after double digestion with BamHI and HindIII, 4 is the DNA marker.
[0099] image 3 The results indicated that the plasmid extracted from the screened Amp-resistant monoclonal...
Embodiment 3
[0100] Example 3 Inducing Genetically Engineered Bacteria to Express Fusion Protein Pro237
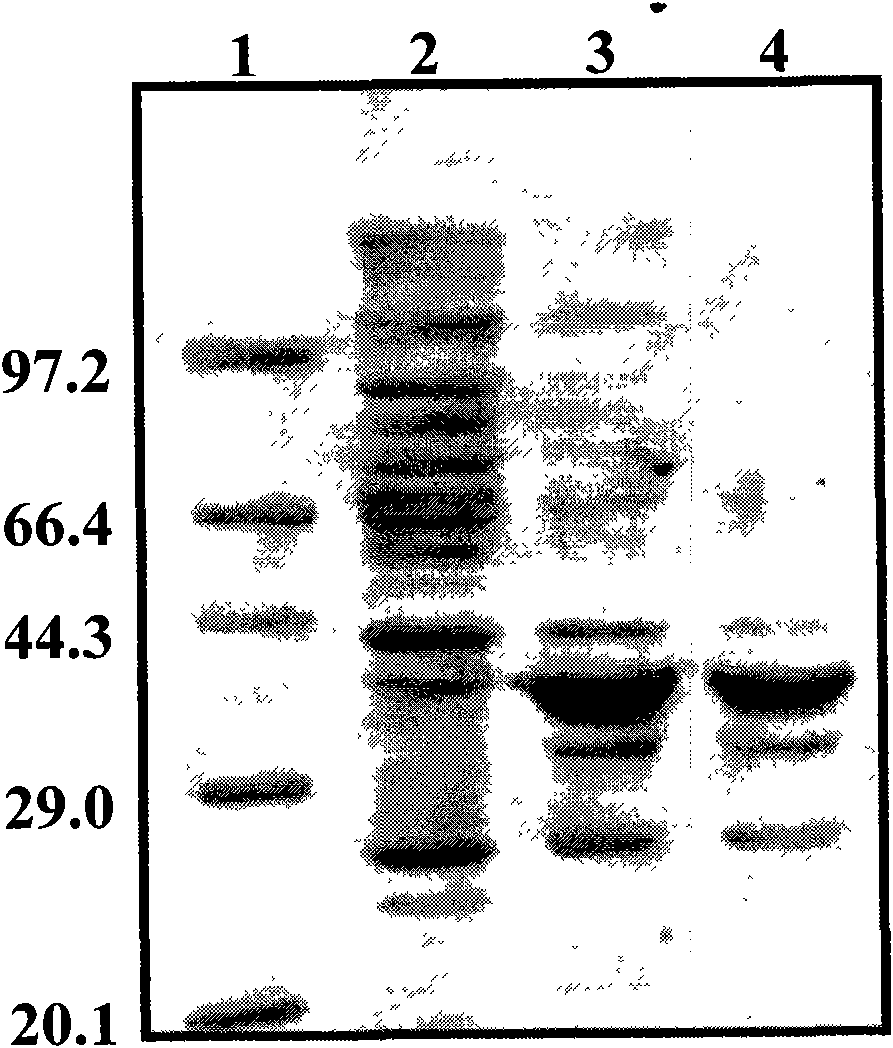
[0101] The recombinant plasmid pET32a-Pro237 was extracted and further transformed into Escherichia coli BL21(DE3) to construct the genetic engineering expression bacteria. Pick a single colony and inoculate it in 5 ml of LB medium containing 100 μg / ml Amp, and cultivate overnight at 37° C. with shaking at 180 rpm. Take 50 μl of the overnight culture and transfer it to 5ml LB medium, culture it at 37°C, 210rpm until the logarithmic growth phase, measure its OD 600 When = 0.5-0.6, add IPTG with a final concentration of 0.1-1 mM to induce expression for 2-4 hours, produce and accumulate soluble expressed Pro237 fusion protein, centrifuge, discard the supernatant, and collect wet cells. (See Figure 4 , Figure 5 )
[0102] Figure 4 It is the SDS-PAGE electrophoresis result graph of the protein expressed by the fusion protein Pro237 induced by IPTG. In the figure, 1 is the low mole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com