Method for preparing non-linear optical metal-organic boron polymer crystal material
A technology of nonlinear optics and crystal materials, applied in cadmium organic compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as weak nonlinear optical effects, and achieve yields that are beneficial to environmental protection, high yields, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Ligand H 3 L
[0031]
[0032] 1,2,4,5-Tetramethylbenzene (10g, 74.6mmol) and iodine (0.394g, 1.56mmol) were dissolved in 380 ml of dichloromethane, and slowly dripped into the light-shielding reactor under nitrogen protection Add bromine (9ml, 175.6mmol) in 40ml of dichloromethane solution (not less than 30 minutes), and reflux for 1 hour after the dropwise addition is complete. After the reaction was completed and returned to room temperature, 20 ml of 5M sodium hydroxide solution was added to quench the reaction. After liquid separation, the organic phase was washed several times with water and dried over anhydrous magnesium sulfate. After drying, the desiccant was filtered off, spin-dried to obtain a white solid, and then the white needle-shaped target product 1,4-dibromo-2,3,5,6-tetramethylbenzene 16.76g (yield : 77%).
[0033]
[0034] Under the protection of nitrogen, 1,4-dibromo-2,3,5,6-tetramethylbenzene (10 g, 34.3 mmol) was dissolved i...
Embodiment 2
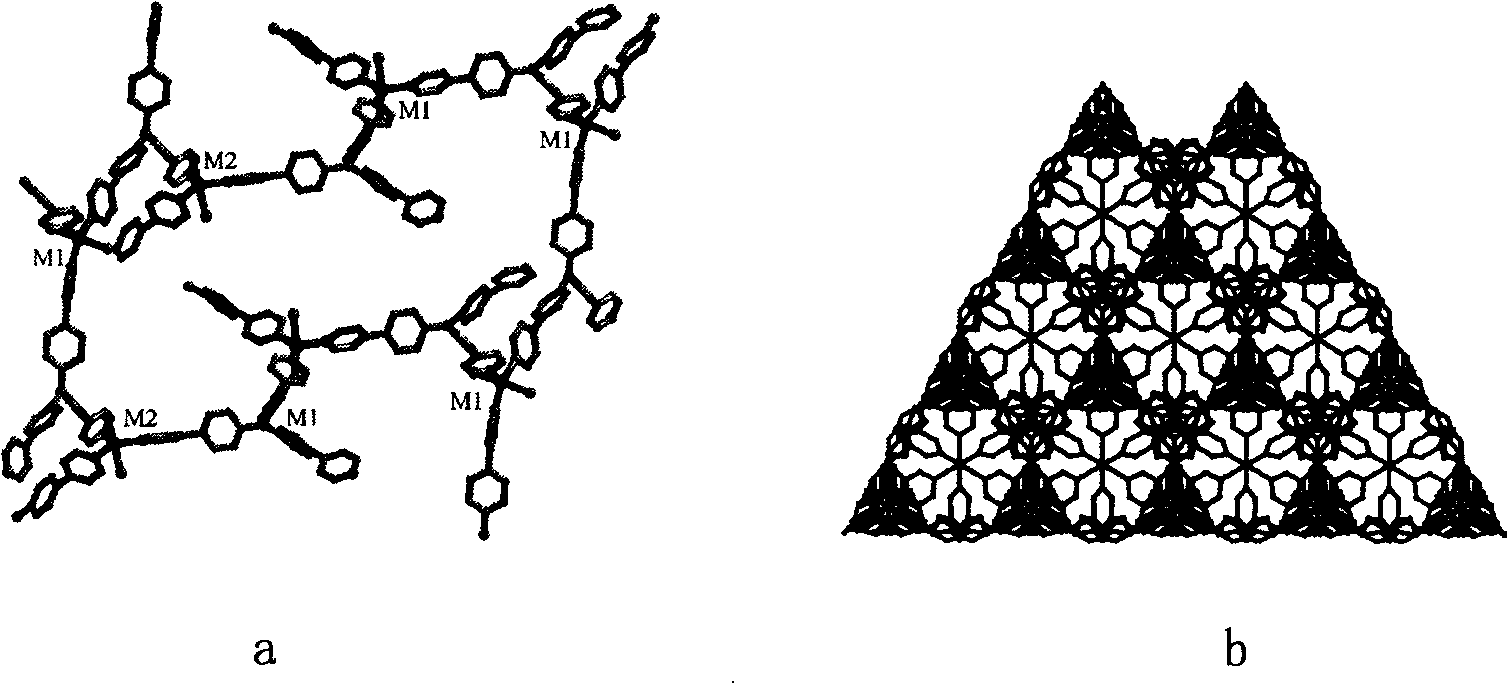
[0040] The preparation of metal organic boron-cadmium chloride polymer crystal material, its SHG effect is shown in the appendix Figure 4
[0041] (1) 2.3mg2.5 cadmium chloride hydrate (CdCl 2 .5 / 2H 2 O) and 3.2 mg ligand H 3 L in 10ml vial;
[0042] (2) Add 2.5 milliliters of ethanol and 0.5 milliliters of toluene in the sample bottle;
[0043] (3) Tighten the bottle cap after fully dissolving, and heat the sample bottle in an oven at 80°C for 2 days;
[0044] (4) Take out the sample bottle after 2 days, and filter the reaction solution after cooling to room temperature;
[0045] (5) The obtained crystals were washed several times with ether and then dried in the air to obtain 2.2 mg homochiral nonlinear optical metal-organoboron polymer crystal material [CdCl 2 L]·2H 2 O, yield 70%.
Embodiment 3
[0047] The preparation of metal organoboron-cadmium iodide polymer crystal material, its SHG effect is shown in the appendix Figure 4
[0048] (1) 3.7mg cadmium iodide (CdI 2 .) and 3.2 mg ligand H 3 L in 10ml vial;
[0049] (2) Add 2.5 milliliters of ethanol and 0.5 milliliters of toluene in the sample bottle;
[0050] (3) Tighten the bottle cap after fully dissolving, and heat the sample bottle in an oven at 80°C for 2 days;
[0051] (4) Take out the sample bottle after 2 days, and filter the reaction solution after cooling to room temperature;
[0052] (5) The obtained crystals were washed several times with ether and then dried in the air to obtain 2.3 mg homochiral nonlinear optical metal-organoboron polymer crystal material [CdI 2 L]·2H 2 O, yield 71%.
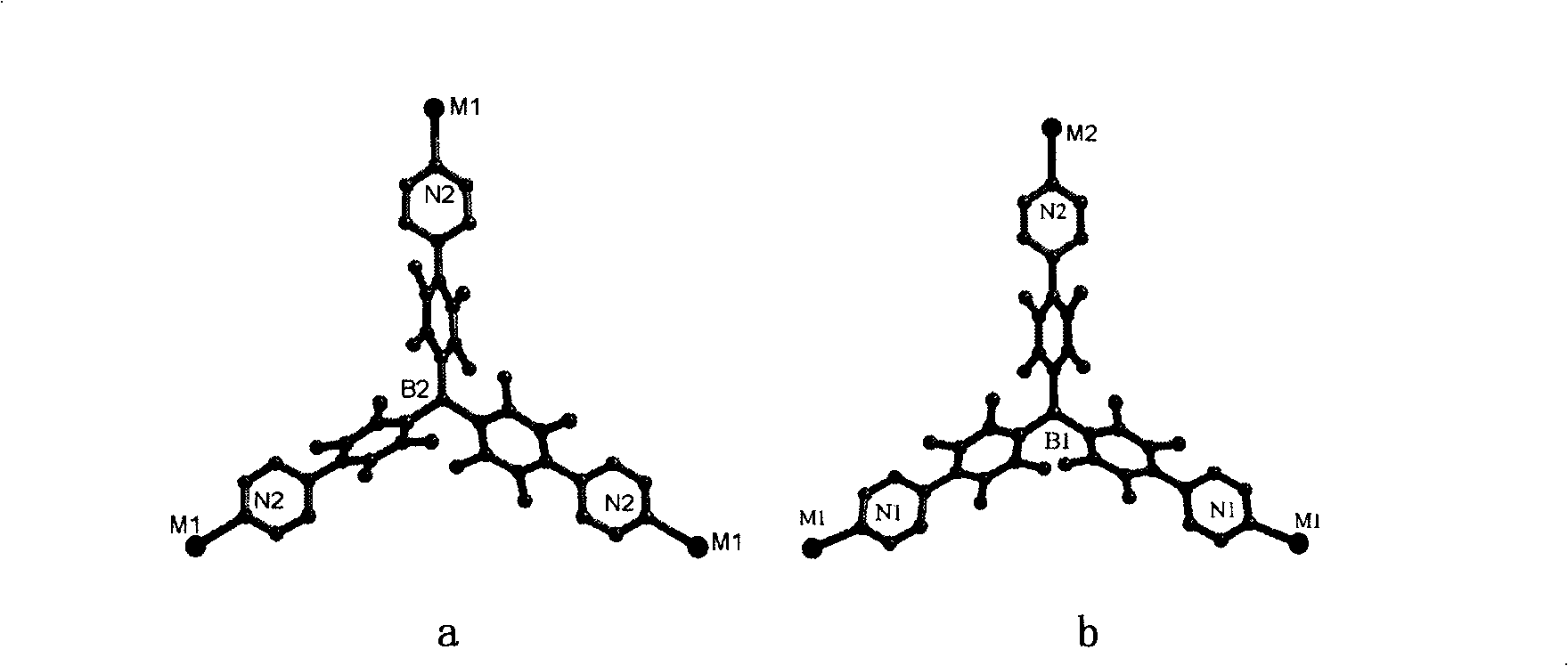
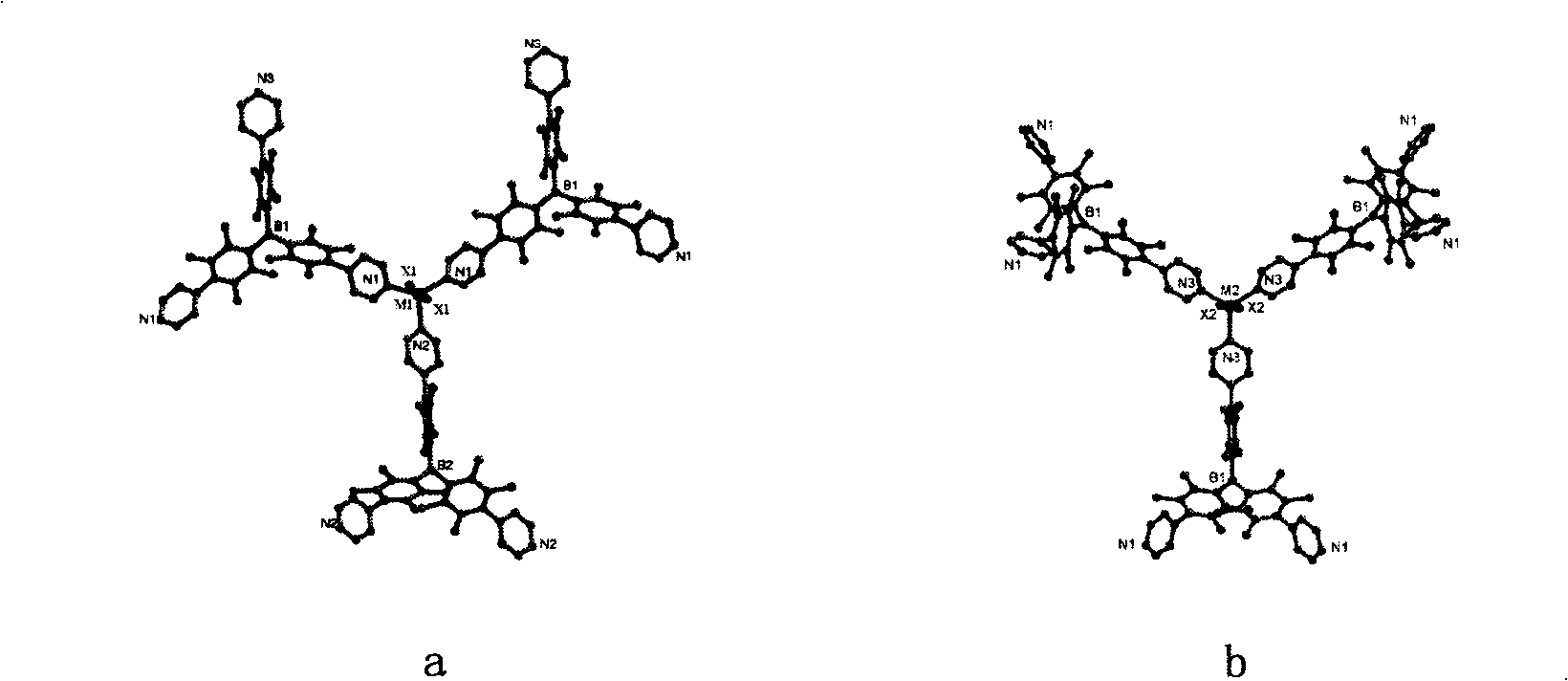
[0053] The specific structure of the obtained crystal obtained by X-ray diffraction shows that the crystal structure formed by the ligand molecule with the center of symmetry after coordinating with the transitio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com