Erythromycin enteric-coated capsule and preparation thereof
An enteric-coated capsule and erythromycin technology, which is used in capsule delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve the problem that unsaturated groups are easily released, affect product quality and efficacy, and cannot guarantee product qualifications. and other problems to achieve the effect of ensuring quality and curative effect, good product quality and high work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] An erythromycin enteric-coated capsule, wherein the proportion of the enteric-coating liquid components of the enteric-coated pellets is calculated by weight: polyacrylic acid resin II: 1, diethyl phthalate: 0.07, Tween-80 : 0.08, castor oil: 0.08, ethanol with a concentration greater than or equal to 95%: 12.
[0025] Its preparation method is as follows:
[0026] a. Dissolve the polyacrylic acid resin II in the ethanol of the above ratio according to the above-mentioned enteric coating liquid composition ratio, and then add the remaining components in the ratio, stir evenly and dissolve to obtain the enteric coating liquid spare;
[0027] b. mixing the erythromycin raw material containing a therapeutically effective amount with sucrose to make erythromycin pellets with a particle size of 0.6 to 0.8 mm for subsequent use;
[0028] c. Enteric-coating the above-mentioned erythromycin pellets: placing the above-mentioned erythromycin pellets in a BZJ-1000F II type coati...
Embodiment 2
[0031] The composition ratio of the enteric coating solution of the enteric-coated pellets is shown in Table 3.
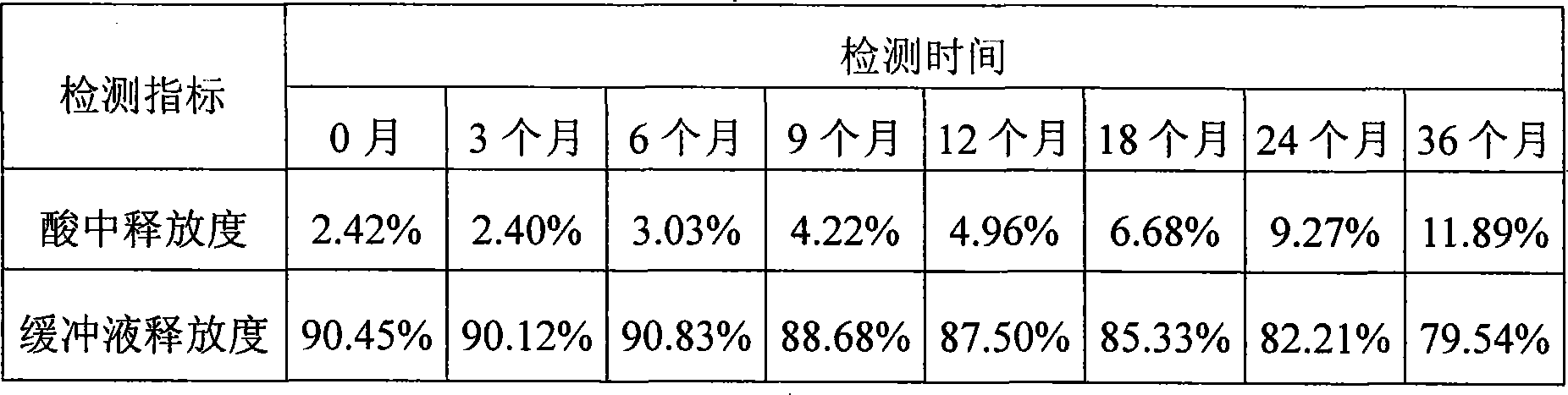
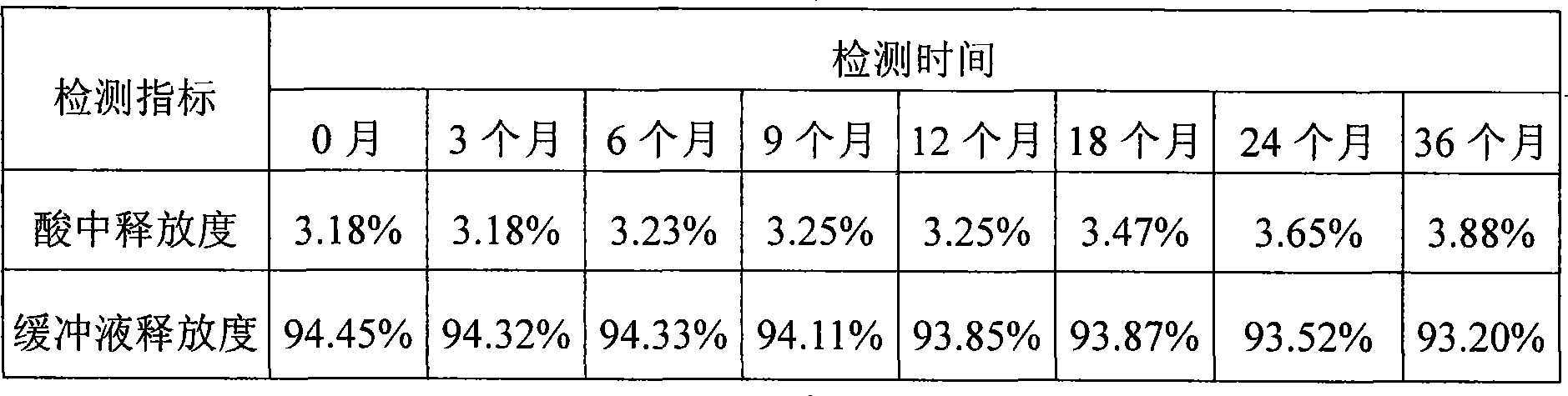
[0032] In the preparation method: in step c, the rotating speed of the coating granulator is 40 rpm, the rotating speed of the spray gun power gear pump is 30 rpm, and 3 blowing devices are added above the coating granulator, and the obtained product is detected in step d. The enteric coating weight gain rate of erythromycin enteric-coated pellets was 12%.
[0033] All the other are with embodiment 1.
Embodiment 3
[0035] The composition ratio of the enteric coating solution of the enteric-coated pellets is shown in Table 3:
[0036] In its preparation method: in step c, the rotating speed of the coating granulator is 60 rpm, the rotating speed of the spray gun power gear pump is 40 rpm, and 2 blowing devices are added around the coating granulator, and the obtained product is detected in step d. The enteric coating weight gain rate of erythromycin enteric-coated pellets was 18%.
[0037] All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com