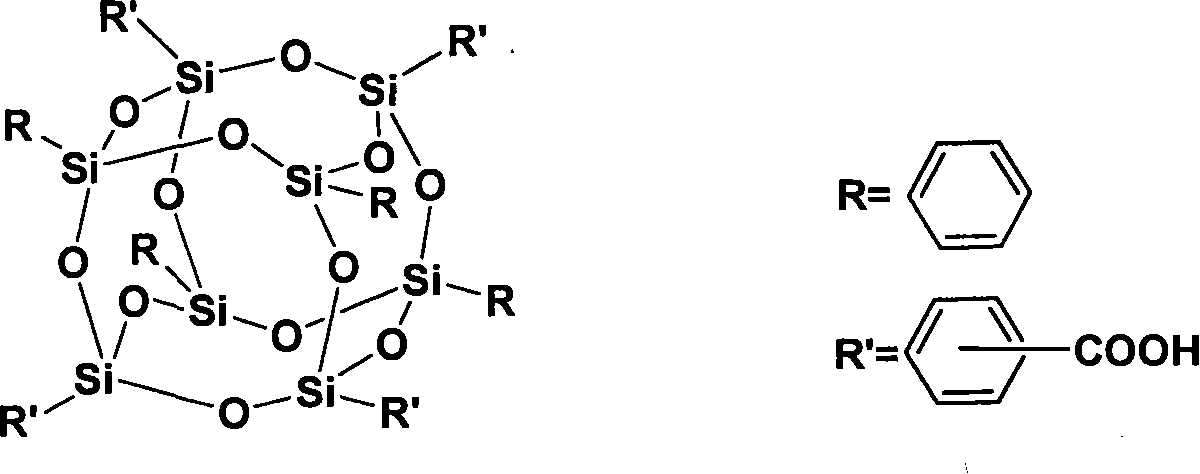
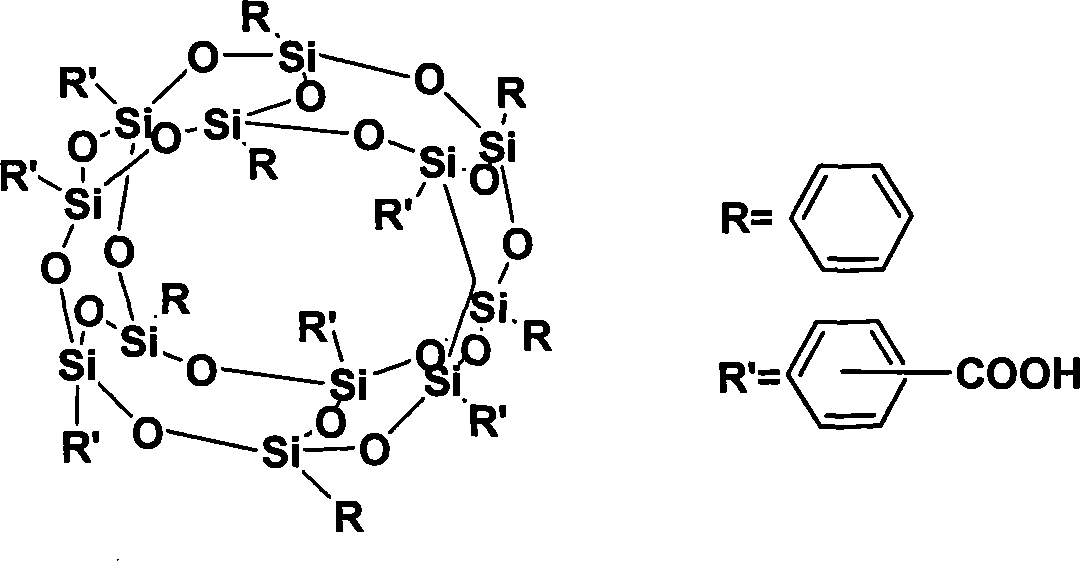
Polycarboxylic cage type phenyl sesquialter siloxane and method for synthesizing same
A technology of phenyl silsesquioxane and silsesquioxane, which is applied in the field of nanomaterials, can solve the problems that the synthesis method of polycarboxy cage silsesquioxane has not been reported, and achieve controllability, cost reduction, and wide range of synthetic raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Polybrominated cage octaphenylsilsesquioxane (SiO 1.5 ) 8 (C 6 h 5 ) 4 {C 6 h 4 Br} 4 Synthesis:
[0021] Add 30g of dry cage octaphenylsilsesquioxane, 2.85g of Fe and 300ml of CH to a 500ml three-necked flask 2 Cl 2 ; Add 12ml of Br dropwise under mechanical stirring 2 . React at room temperature for 6 hours, add 150ml of 10% NaHSO 3 Stop the reaction. The layers were separated, the organic phase was washed three times with water, and the solid crude product was obtained by rotary evaporation. Dry it, dissolve it in a small amount of ethyl acetate, and settle it with 800ml of methanol to obtain a large amount of white precipitate; repeat the precipitation two to three times. After drying, 38 g of white powder was obtained, which was polybromocage octaphenylsilsesquioxane, and the yield was 90%.
Embodiment 2
[0023] Polycarboxy cage octaphenylsilsesquioxane (SiO 1.5 ) 8 (C 6 h 5 ) 4 {C 6 h 4 COOH} 4 Synthesis:
[0024] In a dry 250ml three-necked flask, under nitrogen protection, add 12g (bromine 35.2mmol) (SiO 1.5 ) 8 (C 6 h 5 ) 4 (C 6 h 4 Br) 4 With 80ml of freshly distilled THF; stir mechanically until the temperature reaches -75°C and is stable; slowly add 75ml of Bu-Li (1.6M) dropwise, after the addition is complete and stir for 2.5 hours, slowly add 50ml of 1M HCl dropwise to terminate the reaction. NaOH was added to the water layer to obtain a transparent solution, and concentrated hydrochloric acid was added, a large amount of white solid precipitated, filtered and washed with water 3-5 times. 6.89 g of white solid powder was obtained as polycarboxy cage octaphenylsilsesquioxane (-COOH=4), with a yield of 64%.
Embodiment 3
[0026] Polycarboxy cage octaphenylsilsesquioxane (SiO 1.5 ) 8 (C 6 h 5 ) 4 {C 6 h 4 COOH} 4 Synthesis:
[0027] In a dry 250ml three-neck flask under the protection of nitrogen, carefully add 0.977g (-Br:Bu-Li=1:4.0) metal lithium flakes to 100ml of freshly distilled ether, and mechanically stir until the temperature reaches -10°C and is stable; Slowly add 12g (SiO 1.5 ) 8 (C 6 h 5 ) 4 (C 6 h 4 Br) 4 (bromo group 35.2mmol) and 20ml of freshly distilled ether solution, the time is 3 hours, after the dropwise addition is completed and stirred for 5 hours, dry CO2 Gas for 10 to 12 hours; slowly drop 50ml of ethanol to terminate the reaction. NaOH was added to the water layer to obtain a transparent solution, and concentrated hydrochloric acid was added, a large amount of white solid precipitated, filtered and washed with water 3-5 times. 5.60 g of white solid powder was obtained, which was polycarboxy cage octaphenylsilsesquioxane (-COOH=4), and the yield was 52%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com