Method for low temperature preparing lithium ion battery positive pole material phosphoric acid vanadium lithium
A technology for lithium-ion batteries and positive electrode materials, applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve poor conductivity and cycle performance, uneven particle size distribution of synthetic materials, uneven particle size distribution of materials, etc. problems, to achieve the effect of easy control, inhibition of excessive growth, and simple and convenient methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
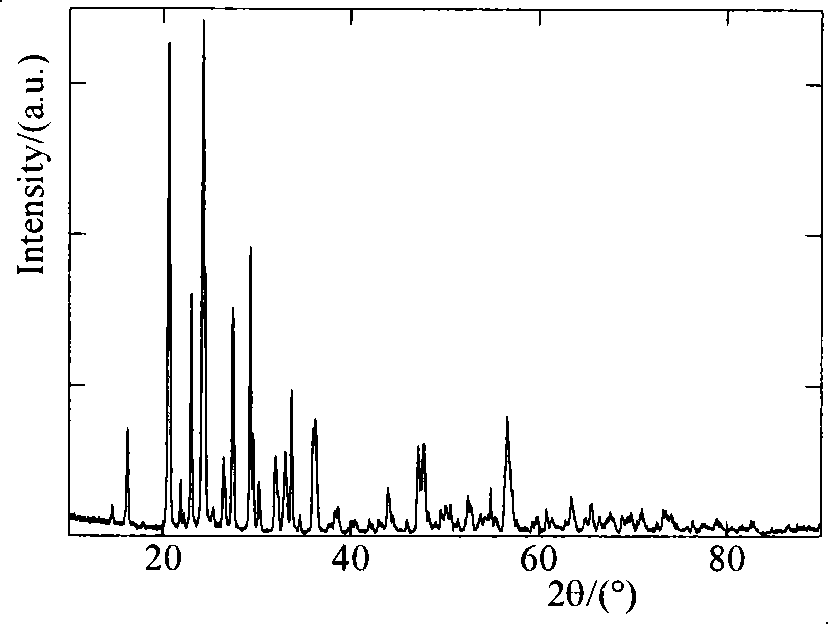
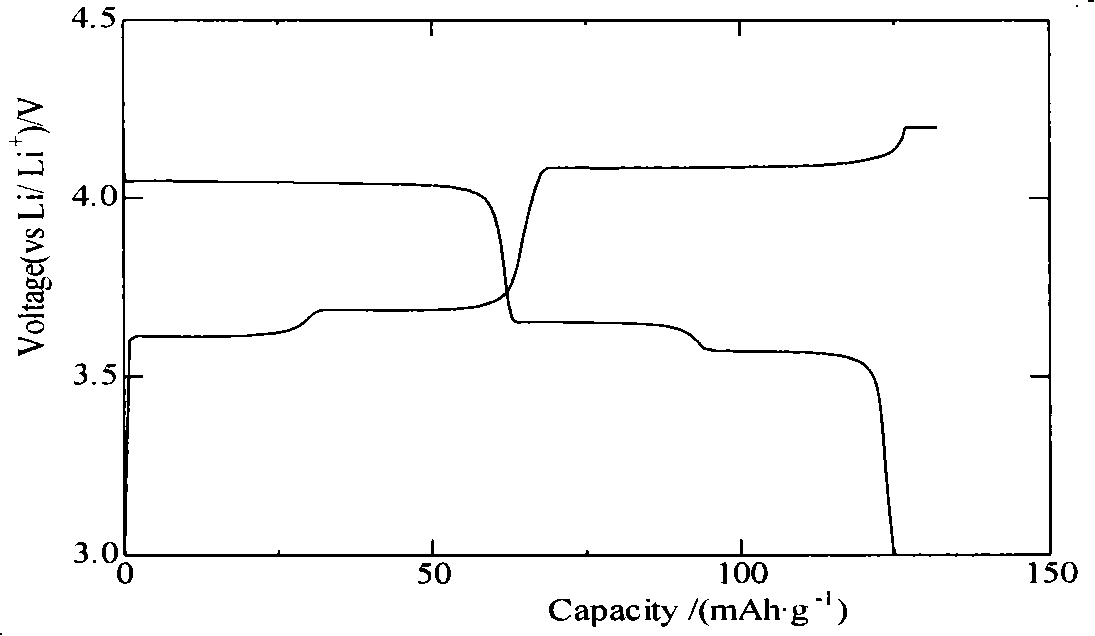
[0016] Heat 0.1mol vanadium pentoxide powder to 600°C, keep the temperature for 4 hours to make it melt, and then quickly pour it into a container filled with water to form a brownish red solution, which can be formed after standing for 16 hours. 2 o 5 ·nH 2 O wet gel. The wet gel was washed to remove most of the moisture, then vacuum-dried at 70° C. for 16 hours, and ground to obtain vanadium pentoxide gel powder. Mix the prepared vanadium pentoxide gel powder with 0.3mol lithium acetate, 0.3mol diammonium hydrogen phosphate and 0.36mol acetylene black, and then sinter at 500°C, 600°C, 700°C, and 800°C under nitrogen protection 10h, after cooling, it is the finished product Li 3 V 2 (PO 4 ) 3 . The obtained products were analyzed by X-ray diffraction, showing that they were all Li 3 V 2 (PO 4 ) 3 , without any impurity, the particle size of the product obtained by SEM is about 1 μm. The obtained product was assembled into an experimental button battery to measure ...
Embodiment 2
[0020] Heat 0.1mol vanadium pentoxide powder to 900°C, keep the temperature constant for 1 hour to melt the vanadium pentoxide, then quickly pour it into a container filled with water to form a brownish-red solution, and leave the solution for 12 hours to form V 2 o 5 ·nH 2 O wet gel. Wash the wet gel to remove most of the moisture, then vacuum-dry it at 90° C. for 12 hours, and grind it to obtain vanadium pentoxide gel powder. After mixing the prepared vanadium pentoxide gel powder with 0.3mol lithium fluoride, 0.3mol ammonium dihydrogen phosphate and 0.36mol acetylene black, they were sintered at 700°C under the protection of argon for 10, 20, 30 and 40 hours respectively. , after cooling, the finished product Li 3 V 2 (PO 4 ) 3 . The obtained products were analyzed by X-ray diffraction, showing that they were all Li 3 V 2 (PO 4 ) 3 , without any impurity, the particle size of the product obtained by SEM is about 1 μm. The obtained product was assembled into an e...
Embodiment 3
[0024] Heat 0.1mol vanadium pentoxide powder to 800°C, keep the temperature constant for 2 hours to melt it, then quickly pour it into a container filled with water to form a brown-red solution, and the solution can be formed after standing for 4 hours. 2 o 5 ·nH 2 O wet gel. The wet gel was washed to remove most of the water, then vacuum-dried at 100° C. for 8 hours, and ground to obtain vanadium pentoxide gel powder. Mix the prepared vanadium pentoxide gel powder with 0.3mol of lithium chloride, 0.3mol of potassium phosphate and 0.36mol of acetylene black, and heat them at 550°C, 650°C, 750°C, and 800°C under the protection of argon. After sintering for 40 hours, the finished product Li is obtained after cooling. 3 V 2 (PO 4 ) 3 . The obtained products were analyzed by X-ray diffraction, showing that they were all Li 3 V 2 (PO 4 ) 3 , without any impurity, the particle size of the product obtained by SEM is about 1 μm. The obtained product was assembled into an e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com