Method for producing electrolytic nickel using various nickel-containing raw material
A technology of electrolytic nickel and raw materials, which is applied in the direction of photographic process, instrument, photographic auxiliary process, etc., can solve the problems of complicated production process of nickel renewable resource recovery, increased consumption of precipitant and extractant, and increased burden of solution purification, etc. Achieve the effects of not being limited by the production scale, flexible production methods, and balanced nickel ion concentration and acid concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
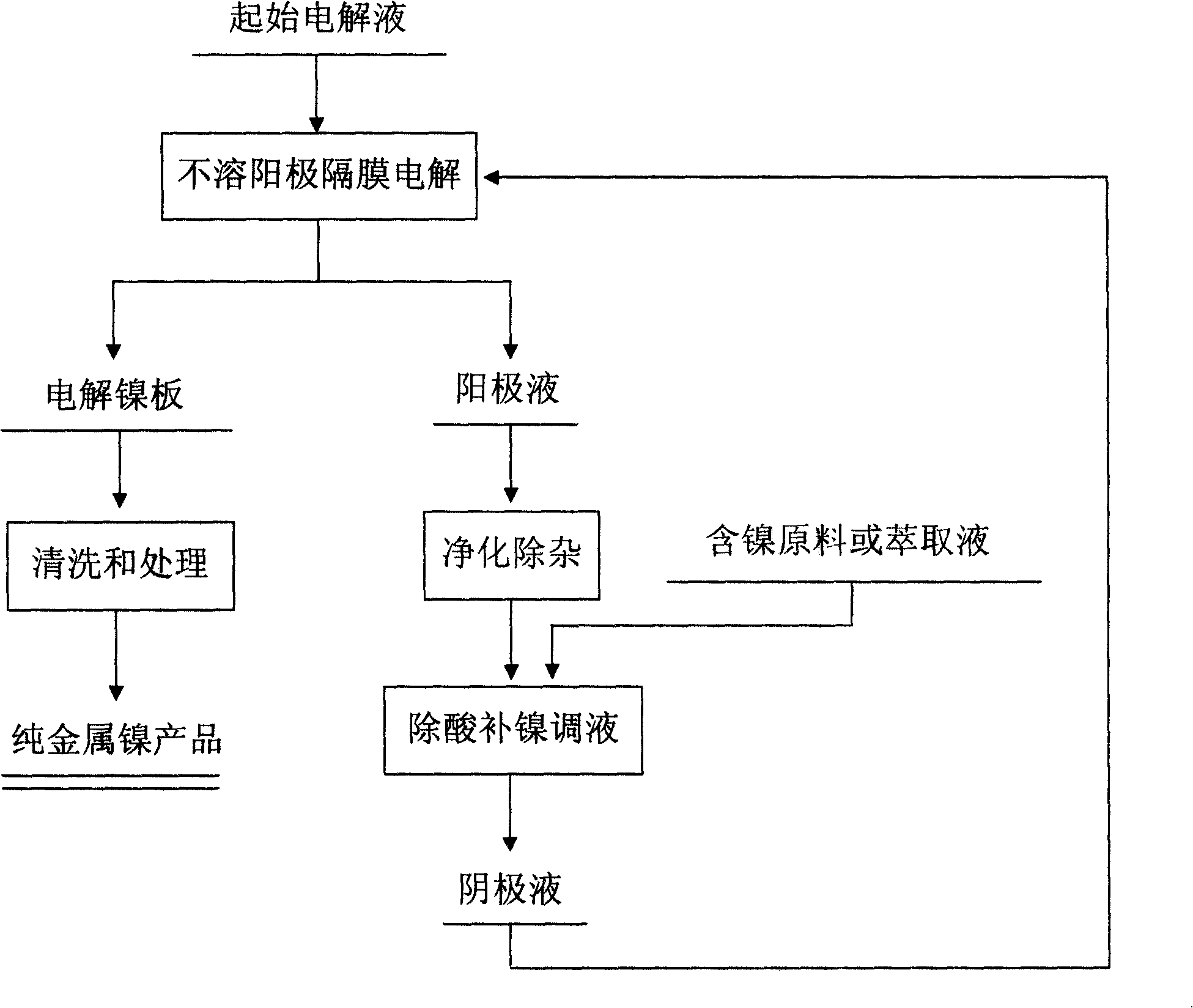
[0036] Example 1: Production of metallic nickel by electrolysis and direct neutralization with an insoluble anode diaphragm. The initial electrode of the nickel plate is used as the cathode, and the anode plate is made of pure lead or lead alloy material. According to the composition requirements of the catholyte, boric acid (1-25g / L), sodium sulfate (70-150g / L), pure nickel sulfate (50 ~120g / L) and other trace additives, adding an appropriate amount of sulfuric acid to adjust the pH value to about 2.0-5.5, after the composition is qualified, heat it to about 40-80°C, pump it into the high-level liquid storage tank, and then flow into the electrolysis In the diaphragm bag in the tank, electrolysis starts, and the nickel ions in the solution are reduced to metal and deposited on the cathode to obtain pure metal nickel. Due to the continuous electrochemical reaction of water on the surface of the anode, oxygen is precipitated on the surface of the anode, and the anolyte continuo...
Embodiment 2
[0039] Example 2: Using a solvent extraction method to remove acid and supplement nickel insoluble anode diaphragm electrolysis to produce metallic nickel. Stainless steel is used as the cathode, and the anode plate is made of pure lead or lead alloy material. Boric acid (1-25g / L), sodium sulfate (70-150g / L), pure nickel sulfate (50-120g / L) are added according to the composition requirements of the catholyte. L) and other trace additives, add an appropriate amount of sulfuric acid to adjust the pH value to about 2.0-5.5, and after the ingredients are qualified, pump them into the high-level liquid storage tank, heat it to about 40-80°C, and then flow into the diaphragm bag. The electrolysis starts, and the nickel ions in the solution are reduced to metal and deposited on the cathode to obtain pure metal nickel. The catholyte penetrates into the anolyte through the diaphragm bag, and the anolyte continuously overflows from the electrolyzer, flows out of the electrolyzer, enters...
Embodiment 3
[0042] Embodiment 3: Using nickel hydroxide as a raw material as a neutralizing agent, the direct neutralization method of insoluble anode electrolysis is used to produce metallic nickel. The titanium plate is used as the cathode, and the anode plate is made of pure lead or lead alloy material. The sulfate system is used to prepare the electrolyte according to the requirements. During the normal diaphragm electrolysis process, the catholyte penetrates into the anolyte through the diaphragm bag, and the anolyte continuously overflows from the electrolytic cell and enters the low-level liquid storage tank. After purification and removal of impurities, the catholyte is pumped into the In the liquid-adjusting mixing tank, the concentration of sulfuric acid in the purified electrolyte changes within the range of 1-60g / L. Add a certain amount of nickel hydroxide into the liquid-adjusting mixing tank, adjust the pH value of the electrolyte to 2.0-5.5, pump the catholyte into the high...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com