Phosphoric acid ligustrazine micropore permeation pump control-release tablet and preparation method thereof
A technology for the controlled release of ligustrazine phosphate and osmotic pumps, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., and can solve the problem of affecting the drug release rate of osmotic pump tablets, inapplicability of mechanical drilling, and membrane burning. Aperture and other issues, to avoid the "peak valley" phenomenon, improve the time of drug action, the effect of low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
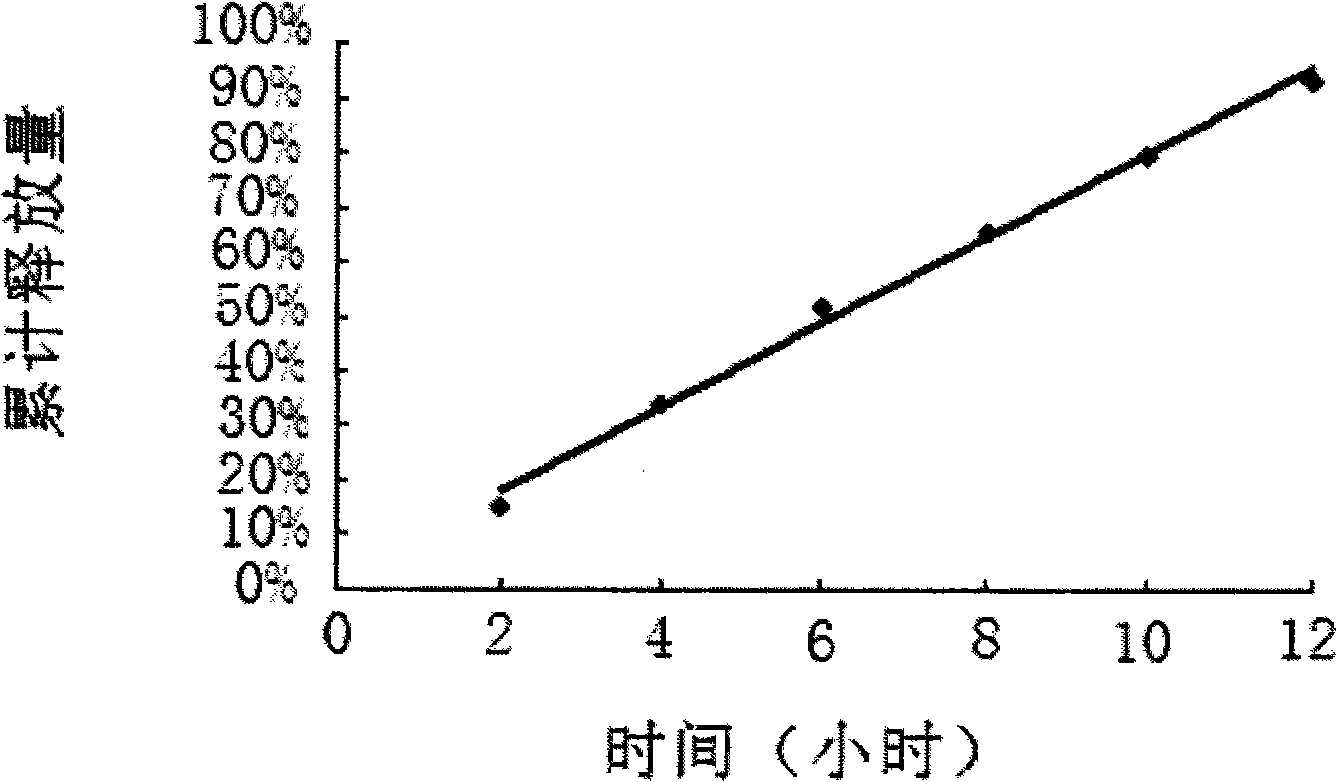
Embodiment 1
[0038] Example 1 is in accordance with the following formula:
[0039]The tablet core weighs 350mg in total:
[0040] Ligustrazine Phosphate 75mg
[0042] Lactose 120mg
[0043] PVP 30mg
[0044] 20% PVP anhydrous ethanol solution
[0045] Magnesium stearate 1.8mg
[0046] Among them, the 20% PVP absolute ethanol solution can be used in accordance with the conventional use in the field to achieve the basic adhesion of the tablet core material. The sodium chloride content can range from 0 to 240 mg, and correspondingly, the lactose content can range from 240 to 0 mg.
[0047] The coating solution is according to the following formula:
[0048] Cellulose acetate 30g
[0049] PEG-400 12ml
[0050] Diethyl phthalate 9ml
[0051] Acetone 800ml
[0052] Isopropanol 200ml
[0053] Coating weight gain 3.5%
[0054] Specific preparation method:
[0055] 1. Weigh 75g of ligustrazine phosphate, 120g of sodium chloride, 120g of lactose, 30g of PVP, pass through ...
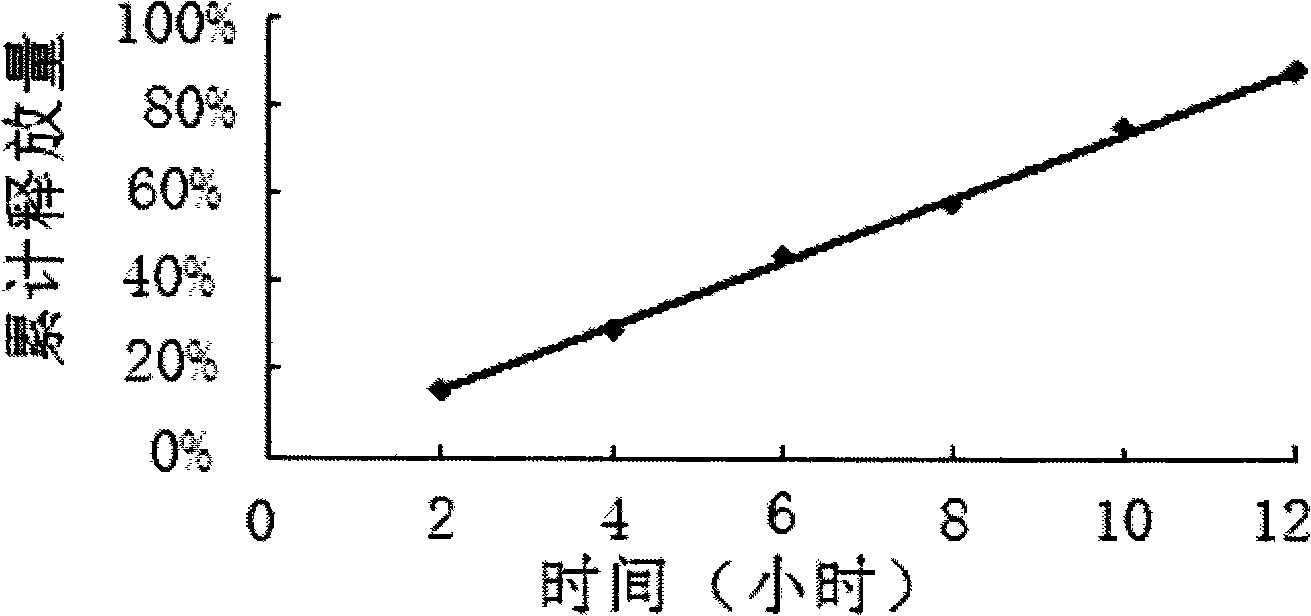
Embodiment 2
[0061] Example 2 is in accordance with the following formula:
[0062] The tablet core weighs 350mg in total:
[0063] Ligustrazine Phosphate 75mg
[0064] Sodium chloride 200mg
[0065] Sodium bicarbonate 40mg
[0066] PVP 30mg
[0067] 20% PVP anhydrous ethanol solution
[0068] Magnesium stearate 1.8mg
[0069] Among them, the 20% PVP absolute ethanol solution refers to the conventional usage in this field to achieve the basic adhesion of the core material.
[0070] The coating solution is according to the following formula:
[0071] Cellulose acetate 30g
[0072] PEG-400 12ml
[0073] Diethyl phthalate 9ml
[0074] Acetone 800ml
[0075] Isopropanol 200ml
[0076] Coating weight gain 4%
[0077] Specific preparation method:
[0078] 1. Weigh 75g of ligustrazine phosphate, 240g of sodium chloride, 30g of PVP, pass through a 60-mesh sieve and mix several times until the mixture is complete. Use 20% PVP in anhydrous ethanol as a binder, 20-mesh sieve, wet granulation Dry in an ov...
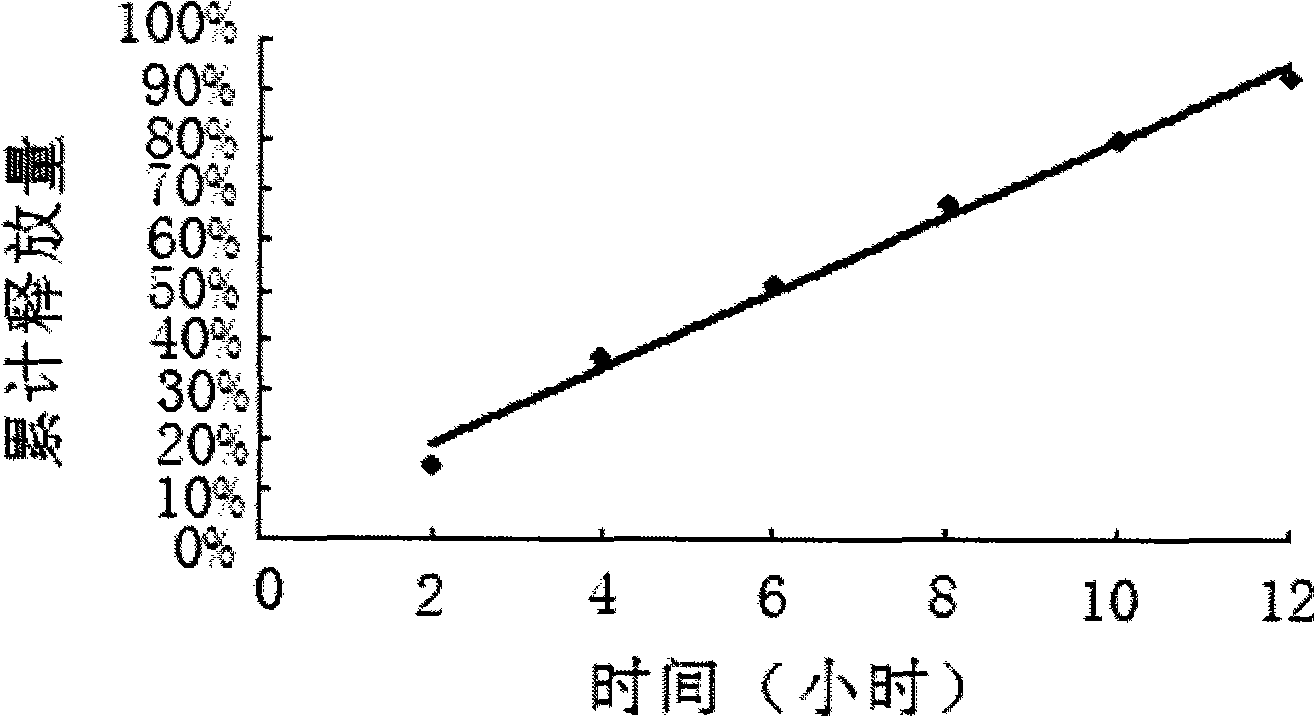
Embodiment 3
[0084] Example 3 is in accordance with the following formula:
[0085] The tablet core weighs 350mg in total:
[0086] Ligustrazine Phosphate 75mg
[0087] Sodium chloride 200mg
[0088] Mannitol 40mg
[0089] PVP 30mg
[0090] 20% PVP anhydrous ethanol solution
[0091] Magnesium stearate 1.8mg
[0092] Among them, the 20% PVP absolute ethanol solution refers to the conventional usage in this field to achieve the basic adhesion of the core material. The sodium chloride content can range from 0 to 240 mg, and correspondingly, the mannitol content can range from 240 to 0 mg.
[0093] The principle of the coating solution formulation is that the solvent uses a mixture of acetone and isopropanol in a ratio of 4:1, the cellulose acetate content is about 3mg / 100ml solvent, and diethyl phthalate accounts for 10-30 of the cellulose acetate content. %, PEG-400 accounts for 10-40% of the cellulose acetate content. The coating liquid of this embodiment is in accordance with the following sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com