Tumour targeting fluorescent probe and application in tumour NO test
A fluorescent probe and tumor-targeting technology, applied in fluorescence/phosphorescence, microbial measurement/inspection, bacteria, etc., can solve the problems of difficult time and space distribution and expression, lack of tumor targeting, etc., and achieve synthesis cost Low, long fluorescence retention time, good practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the synthetic route of probe 1 and probe 2:
[0052] (1) Synthesis of 2-(2-hydroxyphenyl)-benzimidazole: mix equimolar (5~15mmol) salicylaldehyde, sodium bisulfite and 25mL absolute ethanol, stir at room temperature for 3~6h, and then Dissolve equimolar o-phenylenediamine in 25mL DMF, add dropwise to the previous solution and heat to reflux for 2-3h. After the reaction is completed, pour the reactant into 10 times cold water, precipitate out, filter with suction, dry, and recrystallize twice with absolute ethanol;
[0053] (2) Synthesis of 2-(2-hydroxy-5-nitro)-benzimidazole: Dissolve 0.01~0.05g of 2-(2-hydroxyphenyl)-benzimidazole in 10 times the amount of concentrated sulfuric acid and use Cool to -10°C in an ice-salt bath, add dropwise the pre-prepared and cooled mixed acid (concentrated HNO 3 1.0~5.0g; concentrated H 2 SO 4 1.2 to 6.0 g), and keep the temperature at -10°C for 30 to 60 minutes, pour the reactant into ice, filter the precipitate, was...
Embodiment 2
[0060] Example 2: Study on tumor targeting of probe 1 in mice bearing S180 sarcoma
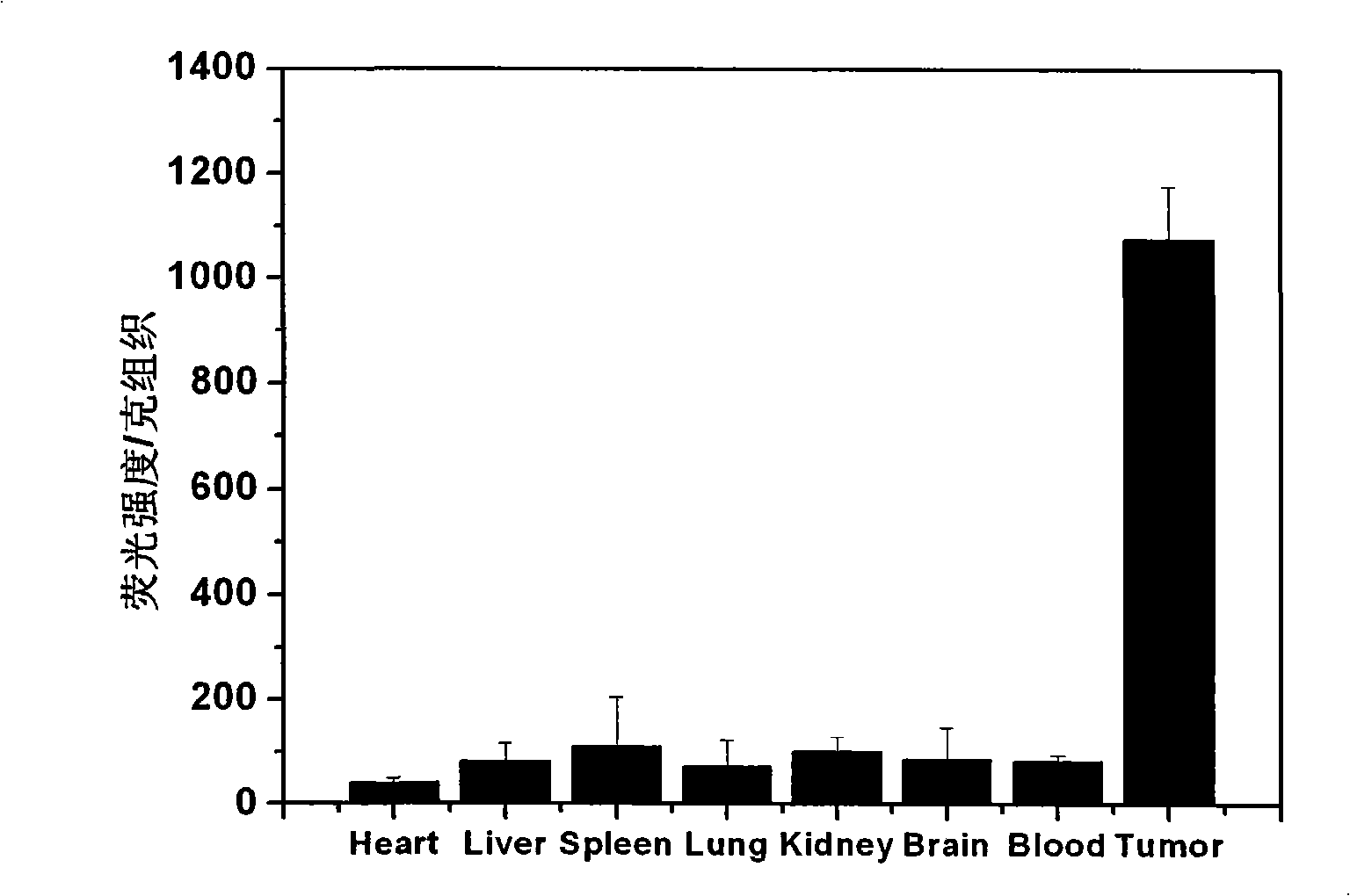
[0061] Inoculate S180 sarcoma cells (at a concentration of 10 6 cells / ml) to the armpit of Balb / c mice (male, 6-8 weeks, 18-22 g), and 200 μl of S180 cells were subcutaneously injected into the armpit of each mouse. One week after inoculation, take 200 μl of the PBS suspension of probe 1 (concentration is 10 9 Top10 / ml), injected into the tail vein of S180 tumor-bearing mice. Two days after the tail vein injection, the mice were sacrificed, and the main organs (heart, liver, spleen, lung, kidney, brain, blood) and tumor tissues were taken, homogenized and dissolved in 800 μl DMSO, and the organ fluorescence distribution of probe 1 was measured , to determine whether it has tumor targeting, when measuring, the excitation light is 330nm, and the fluorescence intensity value at 505nm is measured. Results for each organ are shown as fluorescence intensity per gram of tissue. The results can be...
Embodiment 3
[0064] Example 3: Fluorescence change of probe 2 reacting with NO
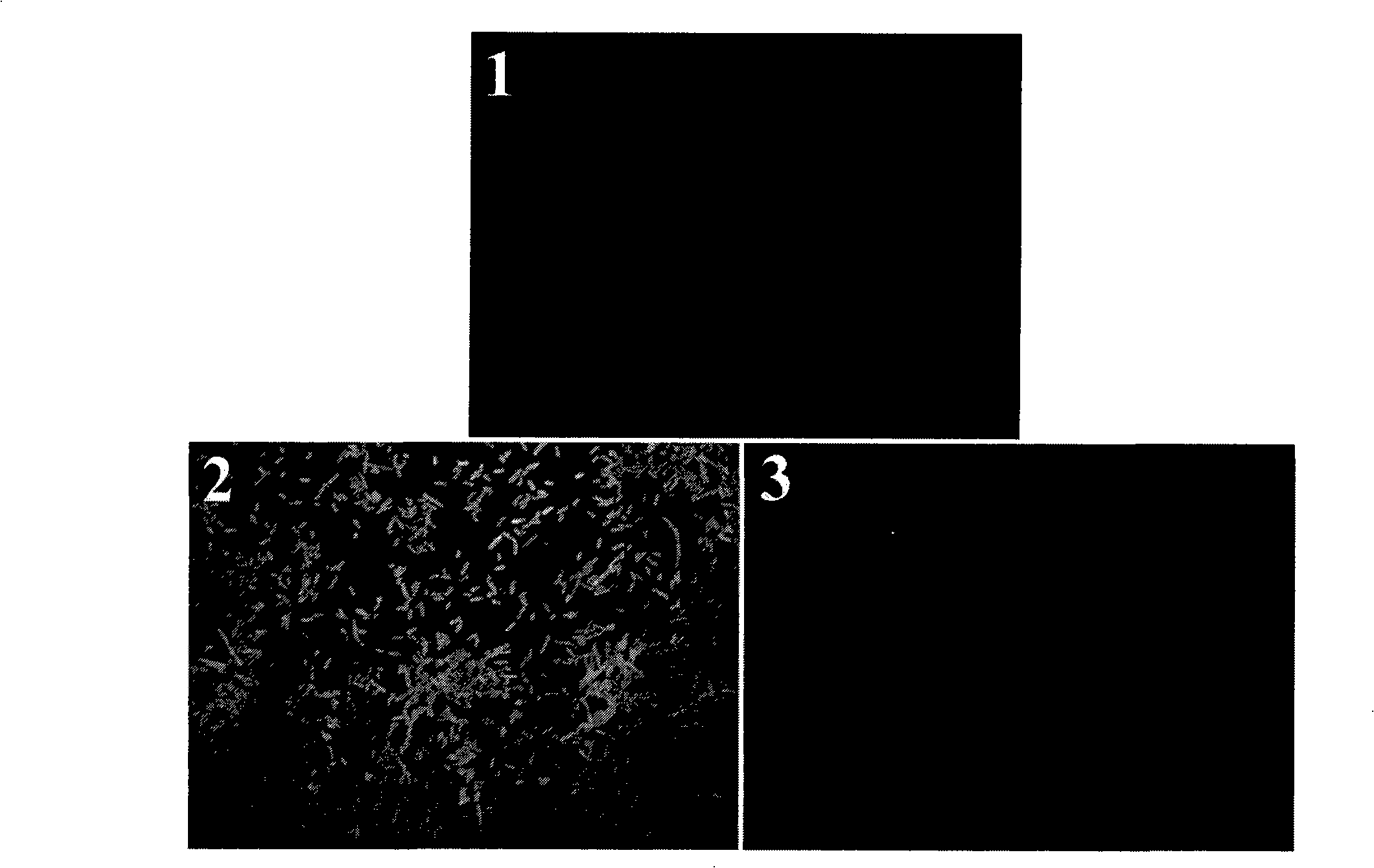
[0065] Suspend Probe 2 in 1×PBS to a final concentration of 10 9 Top10 / ml, take 200μl and react with 1.8mM NO for 1, 5min, measure the change of fluorescence intensity of the suspension, the excitation wavelength is 330nm, see the results Figure 4 , and the fluorescence intensities were normalized. The results of fluorescence photos before and after the reaction between probe 2 and NO can be found in Figure 5 .
[0066] from Figure 4It can be seen from the figure that the background fluorescence intensity of probe 2 is very low. After reacting with NO, the fluorescence intensity rapidly (within 1 minute) increases significantly. After five minutes of reaction, the fluorescence intensity tends to be stable. . The rapid and obvious fluorescence enhancement after reacting with NO indicated that probe 2 could be used as a fluorescent probe for NO detection for the determination of NO. Figure 5 The fluore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com