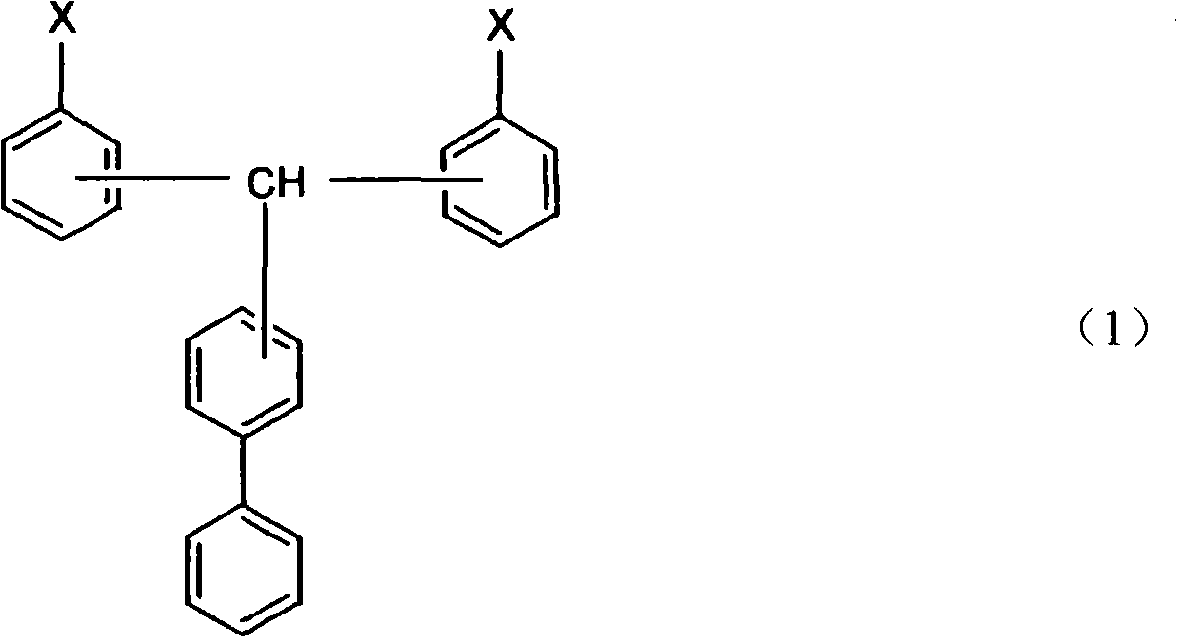
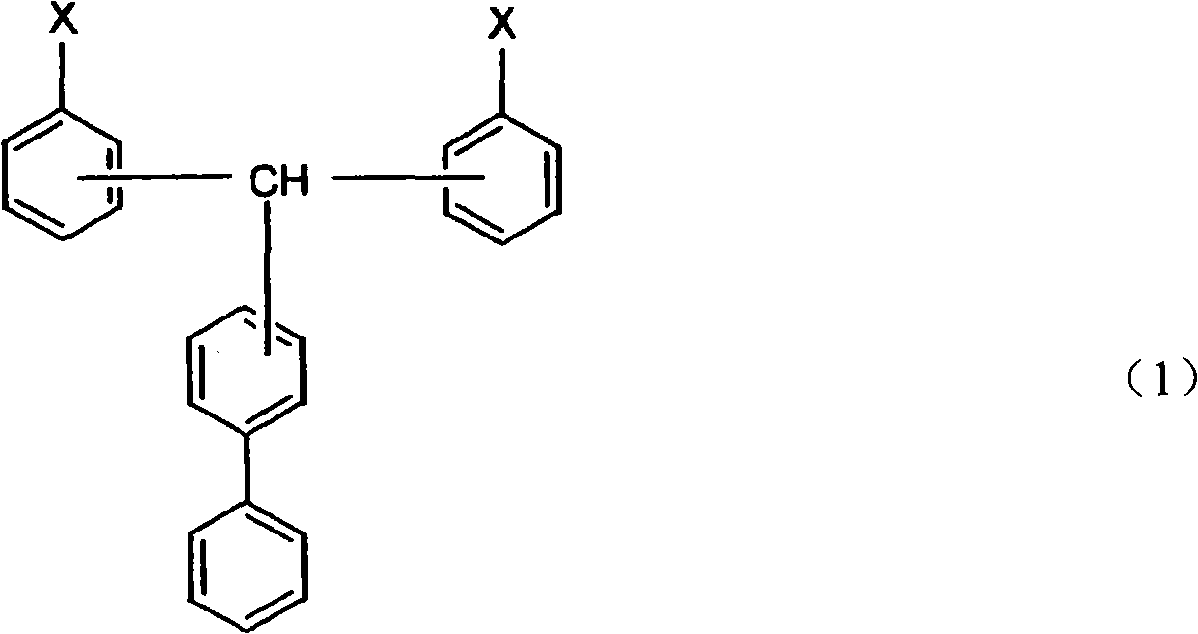
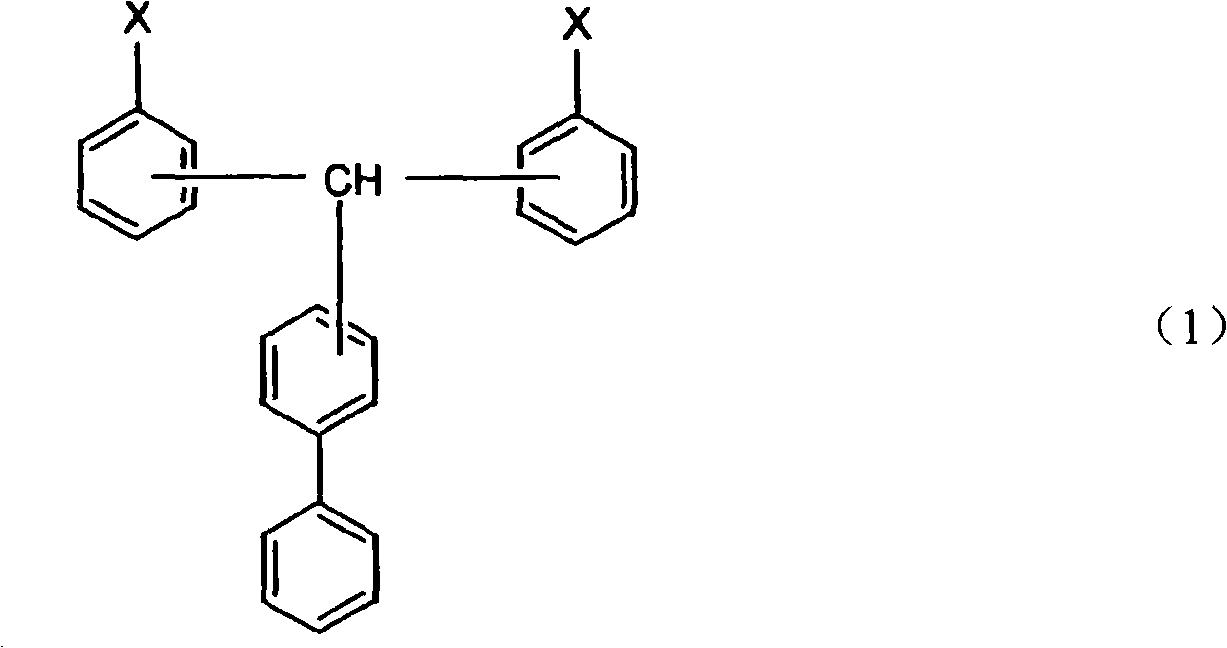
Cyanate ester polymer
A cyanate ester compound and polymer technology, applied in the field of cyanate ester polymers, can solve the problems of low dielectric constant, flame retardancy and heat resistance, and achieve low dielectric constant, low dielectric loss tangent, high The effect of heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The method for preparing the compound represented by the general formula (1) is not particularly limited, and it can be prepared by any existing method for synthesizing cyanate ester. For example, general synthesis methods of cyanate ester compounds are described in IAN HAMERTON, "Chemistry and Technology of Cyanate Ester Resins", BLACKIE ACADEMIC & PROFESSIONAL. In addition, USP3553244 provides a method in which a cyanogen halide is usually reacted in a solvent in the presence of a base in excess relative to the base. Patent No. 3425023 discloses a method of reacting cyanogen halide and basic phenolic resin salt in a cyclic ether solvent, and separating and purifying the product at a temperature below 0°C. Disclosed in No. 7-53497 communiqué of unexamined tertiary amine is as base, and its consumption relative to cyanogen chloride is excessive the method that synthetic cyanate ester is disclosed; The method for the reaction of trialkylamine and cyanogen halide; the sp...
Embodiment A1
[0067] Example A1[Synthesis of cyanate ester (formula (3): abbreviated as BABP-CN) of bisphenol compound of 4-biphenyl formaldehyde]
[0068] [Chem 3]
[0069]
[0070] 0.23 mol of bisphenol compound of 4-biphenyl formaldehyde and 0.51 mol of triethylamine were dissolved in 300 ml of methyl isobutyl ketone (solution 1). Solution 1 was added dropwise to a mixed solution of 200 g of a dichloromethane solution containing 0.60 mol of cyanogen chloride and 1000 g of chloroform at -10° C. over 1.5 hours. After stirring for 30 minutes, a mixed solution of 0.09 mol of triethylamine and 25 g of chloroform was added dropwise, and after stirring for another 30 minutes, the reaction was terminated. The obtained liquid was washed with 1000 ml of 0.1 mol / L hydrochloric acid, and then washed with 1000 ml of water for 4 times. After drying over sodium sulphate and evaporation at 75°C, a reddish-brown sticky substance was obtained. Then, drying under reduced pressure was carried out at 9...
Embodiment B1
[0072] Embodiment B1 [preparation of polymer (cured product)]
[0073] The BABP-CN obtained in Example A1 was weighed into an eggplant-shaped flask according to the ratio in Table 1, heated and melted at 150°C, and degassed with a vacuum pump, then zinc octoate was added, and stirred for 1 minute. Then cast in a mold made of a glass plate (120mm×120mm×5mmt), a polyimide film (Capton 200H: Toray Dupon), and an O-ring made of fluororubber (S-100: Morisei), and Curing was performed by heating in an oven at 170° C. for 1 hour, and then at 230° C. for 9 hours. After cooling, the polyimide film was removed by grinding to obtain a polymer of cyanate compound.
[0074] The performance of the obtained cured product was evaluated by the following method.
[0075] Glass transition temperature (Tg): determined by dynamic viscoelasticity test (DMA). The measurement was performed at a vibration frequency of 10 GHz.
[0076] Permittivity, dielectric loss tangent: calculated by cavity res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com