Preparation method for nano multilayer nickelous hydroxide hollow tube
A multi-layer, hollow tube technology, applied in the field of nanomaterials, can solve the problems of difficult doping, low output, and difficult mass production, etc., and achieve the effect of simple experimental process, cheap and easy-to-obtain raw materials, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
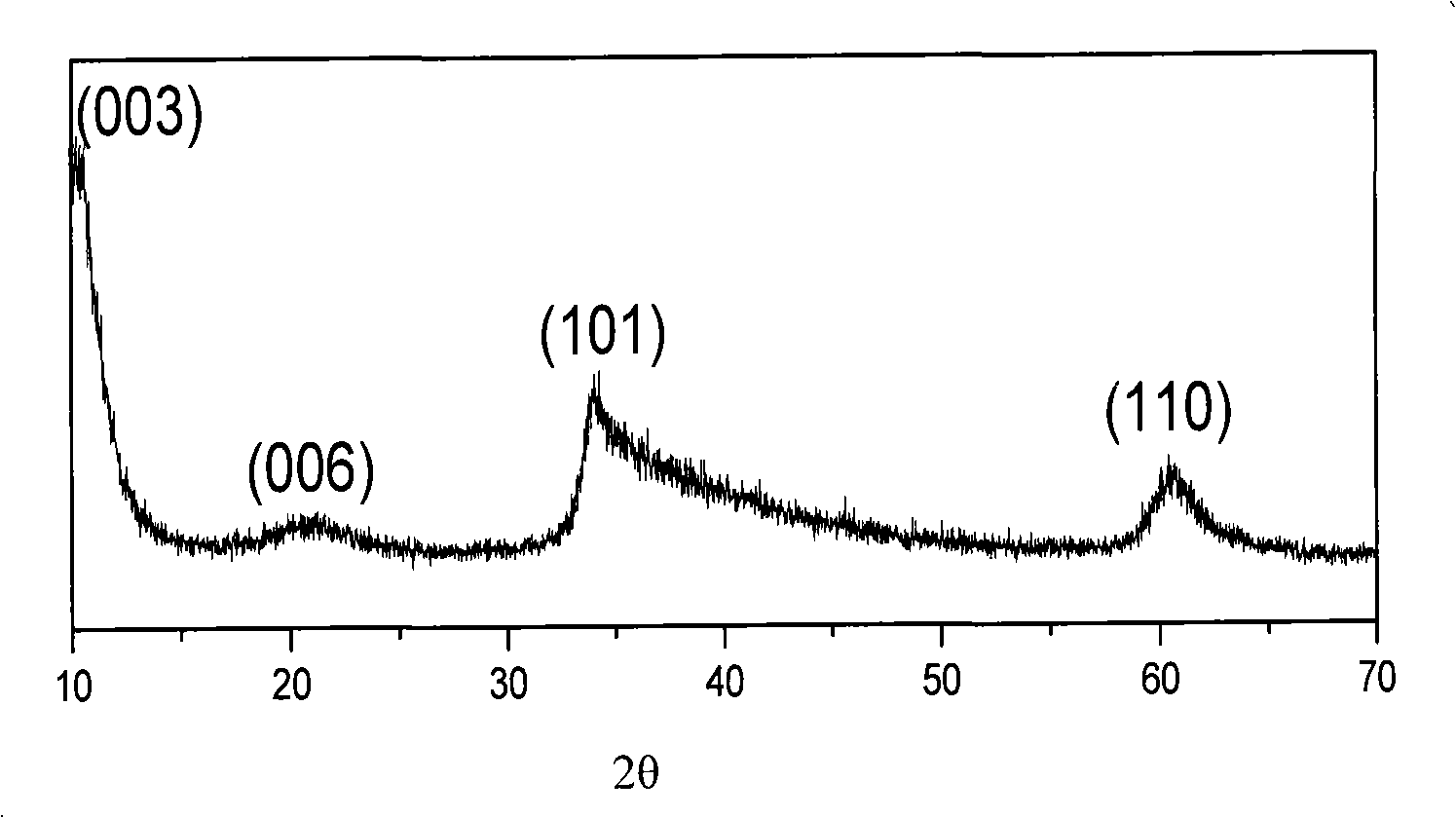
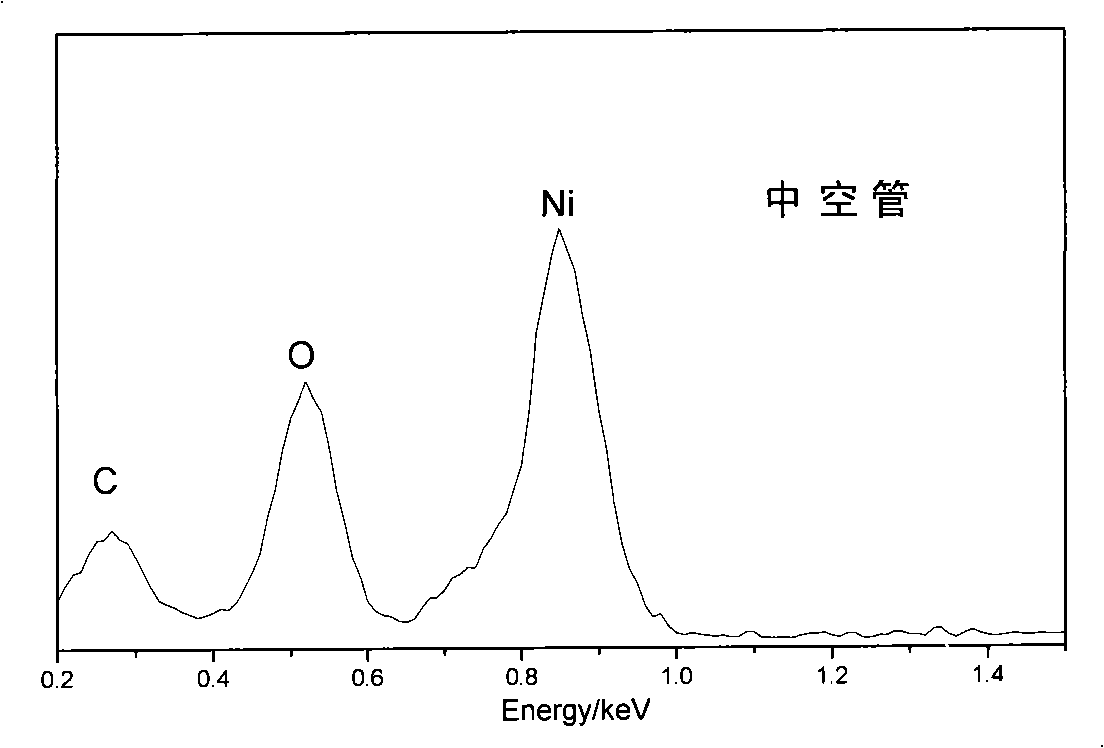
[0021] Prepare 0.001mol nickel chloride hexahydrate and 0.12mol ethylene glycol and put them into a 40mL stainless steel reaction kettle lined with polytetrafluoroethylene, add 0.45g sodium acetate, stir with a glass rod until completely dissolved, and form a light green transparent mixture solution. Close and tighten the reactor, put it into an oven, set the temperature at 190°C, take out the reactor after 3 hours of reaction, and let it cool naturally. Subsequently, the reaction precipitate was poured out, washed three times with distilled water, and then washed three times with absolute ethanol. Finally, it was dried in a vacuum oven at 60°C for 8 hours, and the samples were collected and stored in a desiccator. figure 1 The nano-multilayered α-Ni(OH) prepared for this embodiment 2 The X-ray diffraction spectrum of the hollow tube shows that the obtained powder is α-Ni(OH) with a hydrotalcite structure 2 , the diffraction peaks in the spectrogram correspond to the crysta...
Embodiment 2
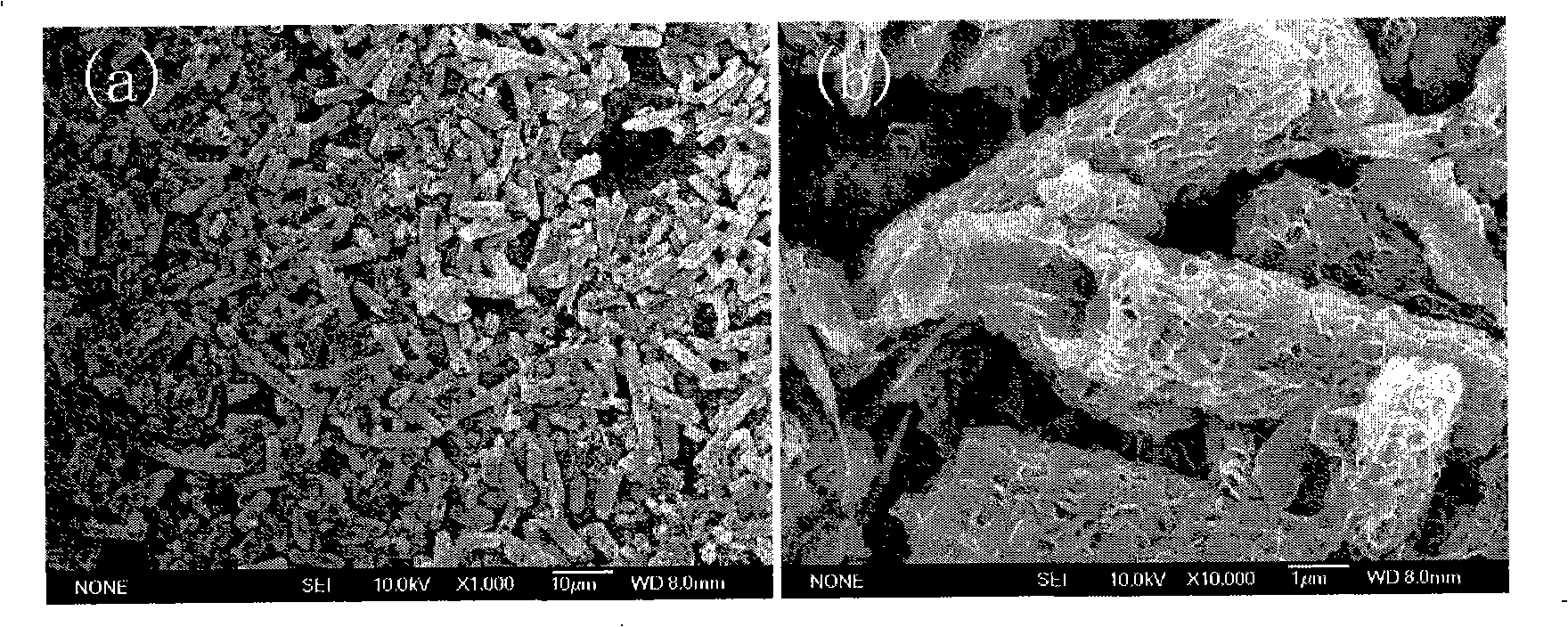
[0023] Prepare the molar ratio of nickel chloride hexahydrate and ethylene glycol as 1:120, put it into a PTFE-lined stainless steel reaction kettle with a volume of 40mL, add 0.45g of sodium acetate, stir with a glass rod until completely dissolved, and form Light green transparent mixed solution. Close and tighten the reactor, put it into an oven, set the temperature at 170°C, take out the reactor after 6 hours of reaction, and let it cool naturally. Subsequently, the reaction precipitate was poured out, washed three times with distilled water, and then washed three times with absolute ethanol. Finally, it was dried in a vacuum oven at 70°C, and the collected samples were stored in a desiccator. TEM image ( Figure 4 a) shows that the outer diameter of the prepared nanotube is 1.6-1.9 μm, and the outer wall of the tube is made of a large amount of soft 30-40nm thick α-Ni(OH) 2 Composed of nanosheets, the aspect ratio can be as high as 10. Figure 4 The inset in a is a si...
Embodiment 3
[0025] Prepare the molar ratio of nickel chloride hexahydrate and ethylene glycol as 1:80, put it into a stainless steel reaction kettle with a polytetrafluoroethylene liner with a volume of 40mL, add 0.45g of sodium acetate, stir with a glass rod until it is completely dissolved, and form Light green transparent mixed solution. Close and tighten the reactor, put it into an oven, set the temperature at 160°C, take out the reactor after 6 hours of reaction, and let it cool naturally. Subsequently, the reaction precipitate was poured out, washed three times with distilled water, and then washed three times with absolute ethanol. Finally, it was dried in a vacuum oven at 70°C, and the collected samples were stored in a desiccator. TEM image ( Figure 4 b) It shows that the outer diameter of the prepared nanotube is 500-600nm, and the outer wall of the tube is composed of a large number of soft, interlaced ~20nm thick α-Ni(OH) 2 Nanosheets are self-assembled.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com