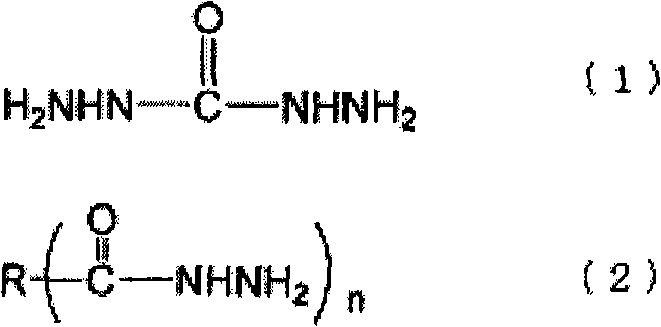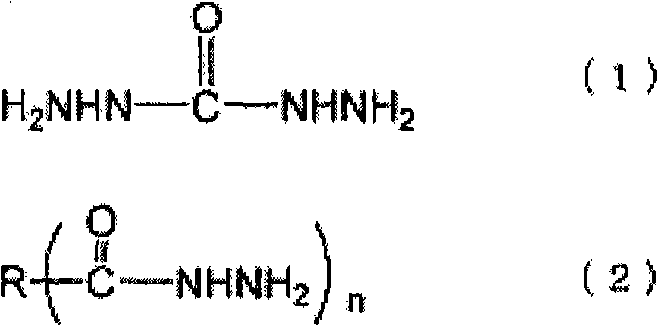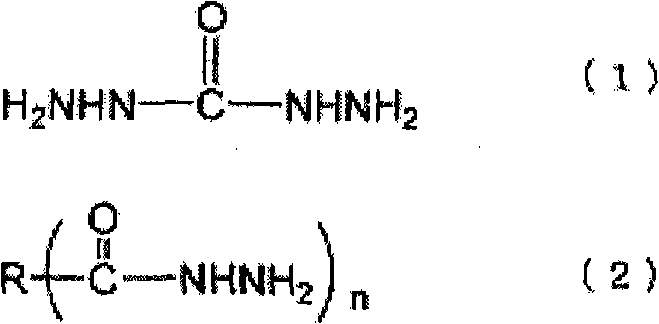Method for producing metal particle dispersion, conductive ink using metal particle dispersion produced by such method, and conductive coating film
A technology of metal particles and a manufacturing method, which is applied to conductive materials dispersed in non-conductive inorganic materials, conductive layers on insulating carriers, inks, etc. and other problems, to achieve the effect of excellent fluidity and stability, low cost, and improved practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] The preparation method of the ammonia complex is not particularly limited, and for example, it can be easily prepared by a known method such as adding ammonia water dropwise while stirring a solution of an inorganic salt compound. The amount of ammonia added in the preparation of the ammonia complex varies depending on the type and concentration of the inorganic salt compound, but as long as the complex can be stably dissolved and the amount of ammonia that is generated when the neutralizing inorganic salt compound is reduced The required stoichiometric ratio of the acid is not particularly limited. For example, with regard to the silver-ammonia complex, if there is no excess ammonia, a brown precipitate will be generated, so be careful, but considering the purification and cost after the reaction, it is preferably 10% to the metal of the inorganic salt compound. Mole times less. More preferably, the ratio of the metal to the inorganic salt compound is 6 mole times or ...
Embodiment 1
[0156]A condenser tube, a thermometer, a nitrogen inlet pipe, and a stirring device are installed on a separable four-necked flask, and 91.1 parts of toluene and 3.2 parts of Solsperse 32000 (manufactured by Japan Lubrizol Co., Ltd.) as a pigment dispersant are dropped into while introducing nitrogen. After the solution was dissolved, 73.1 parts of a 20% succinic acid dihydrazide aqueous solution (2 mole times the hydrazide group relative to 1 mole of metal) was added dropwise while stirring at 50° C. to form uniform droplets. Weigh 100 parts of 1M silver nitrate aqueous solution in a beaker, add dropwise 27.3 parts of 25% ammonia water (4 mole times relative to 1 mole of metal) while stirring, and then add the obtained ammonium complex aqueous solution dropwise to the above toluene solution , and the reaction was carried out at 30 °C. After standing and separating, the excess reducing agent and impurities are removed by taking out the water phase, and then for the toluene pha...
Embodiment 2
[0158] Except having changed the addition amount of the pigment dispersant into 0.5 part, it carried out similarly to Example 1, and obtained the silver fine particle dispersion. The obtained silver microparticle dispersion has fluidity and broad absorption at 429nm. The average particle diameter of the silver microparticles is 15±10nm, showing a wide distribution, and the silver concentration is 50%. The yield of this silver microparticle dispersion was 40%, and the particle size became 50 nm after storage at 40° C. for one month.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com