N-benzoyl rhodamine B hydrazine, preparation and use thereof
A technology for the reaction of benzoyl and benzoyl chloride, which is applied in the fields of fluorescence/phosphorescence, organic chemistry, color/spectral characteristics measurement, etc., can solve the problems of application limitation, poor selectivity, and susceptibility to interference, so as to improve detection sensitivity and selectivity High sensitivity, high detection sensitivity, widening application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
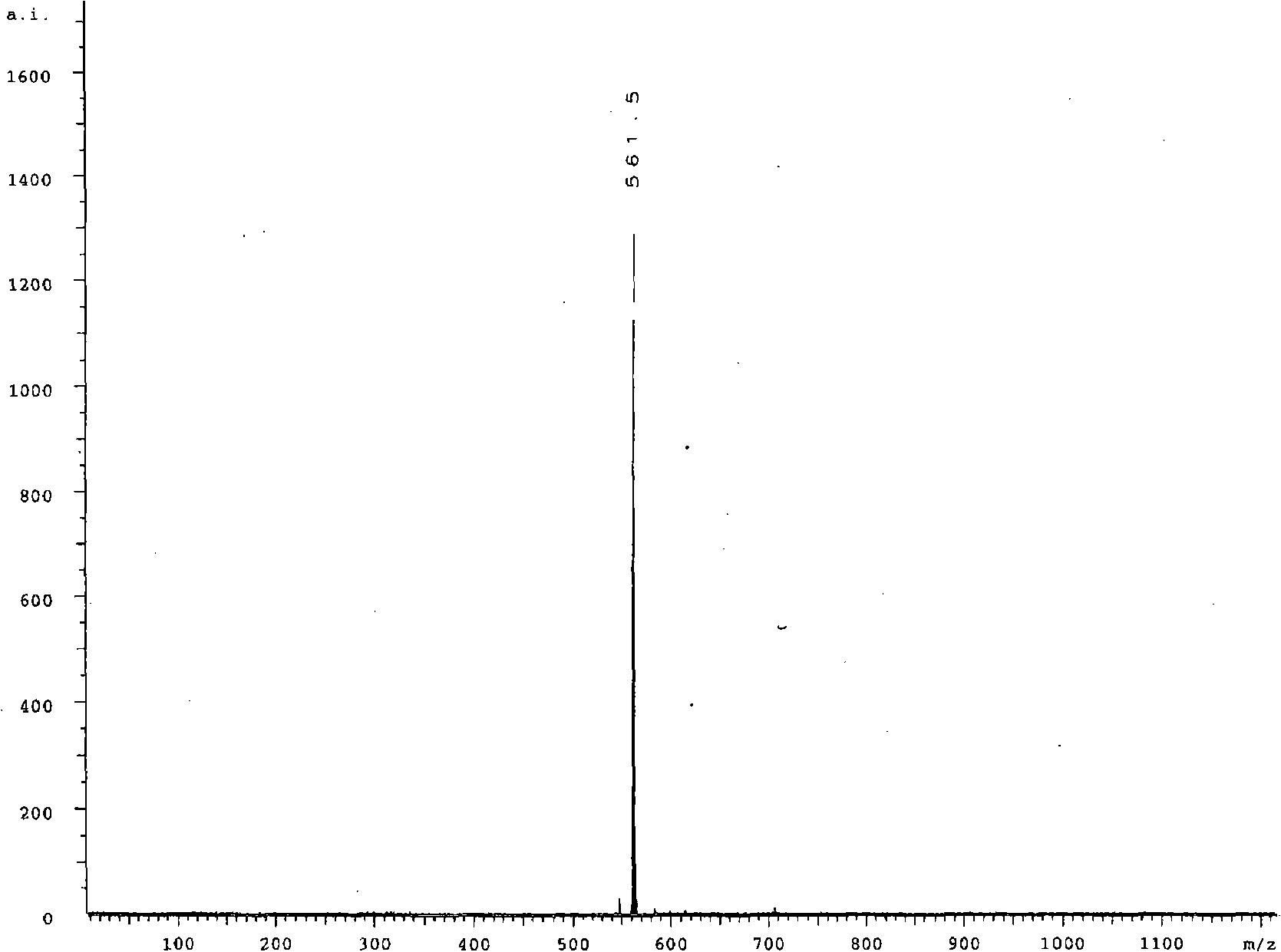
[0016] Example 1. The preparation reaction process of the analytical reagent N-benzoylrhodamine B hydrazine derived from xanthene dye Rhodamine B is represented by the following reaction process:
[0017]
[0018] Rhodamine B (1) (1 g) was dissolved in anhydrous methanol (40 mL) to form a clear solution. To this solution was added excess hydrazine hydrate (85%, 4mL), heated to reflux with stirring for 6h, then the reaction system was removed with a rotary evaporator to remove the solvent, the residue was recrystallized in methanol-water to obtain 750mg of product rhodamine B hydrazine (2 ), the yield is 78.8%.
[0019] Rhodamine B hydrazine (2) (200mg) and triethylamine (400μL) were dissolved in 5mL THF / 1mL water, and benzoyl chloride (3) (300μL) was dissolved in THF (5mL) to form clear solutions. At 0 Under stirring at °C, drop the THF solution of benzoyl chloride into the solution of rhodamine B hydrazine. After the addition is complete, the system is stirred overnight for rea...
Embodiment 2
[0022] Example 2: N-benzoyl rhodamine B hydrazine is used as an analytical reagent to detect hypochlorite by absorption
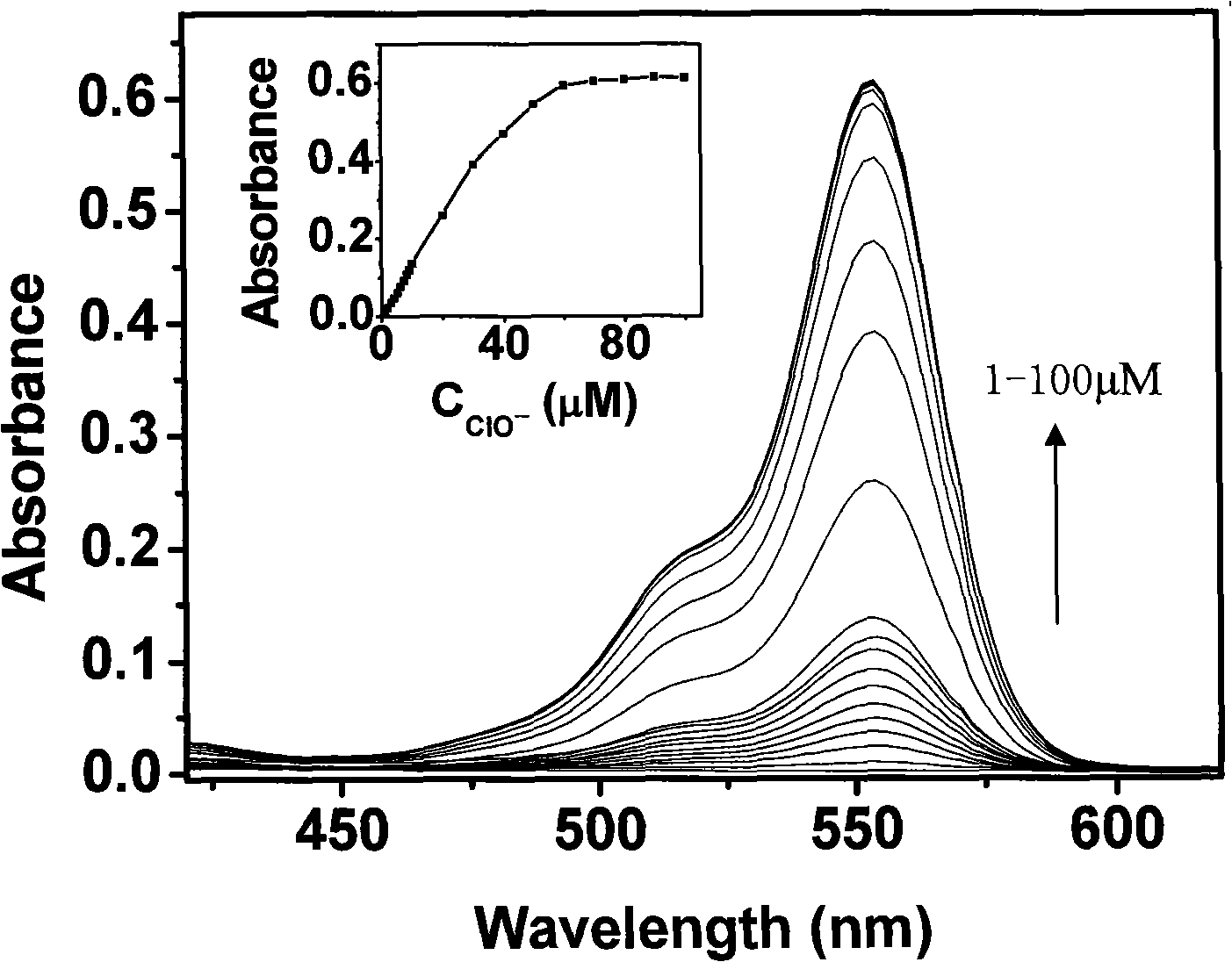
[0023] Add 100μl of reagent—N-benzoylrhodamine B hydrazine in THF (concentration 1mM) to a 10ml test tube, dilute to 3ml with THF, then add 2ml of secondary deionized water and 3ml of 0.1M Na 2 B 4 O 7 -NaOH buffer solution (pH=12.2), finally add different amounts of sodium hypochlorite mother liquor and dilute the volume to 10ml with secondary deionized water (make the sodium hypochlorite concentration in the system 1-100μM respectively), shake and react at room temperature for 30min. Use the same operation but no reagents—N-benzoylrhodamine B hydrazine and sodium hypochlorite solution as a blank to measure the absorption spectrum of the reaction system.
[0024] figure 2 It is the relationship between the concentration of sodium hypochlorite and the absorption spectrum of the reaction system (inset figure: the linear relationship between the absorbance of t...
Embodiment 3
[0030] Example 3. Fluorescence detection of hypochlorite with N-benzoyl rhodamine B hydrazine as an analytical reagent
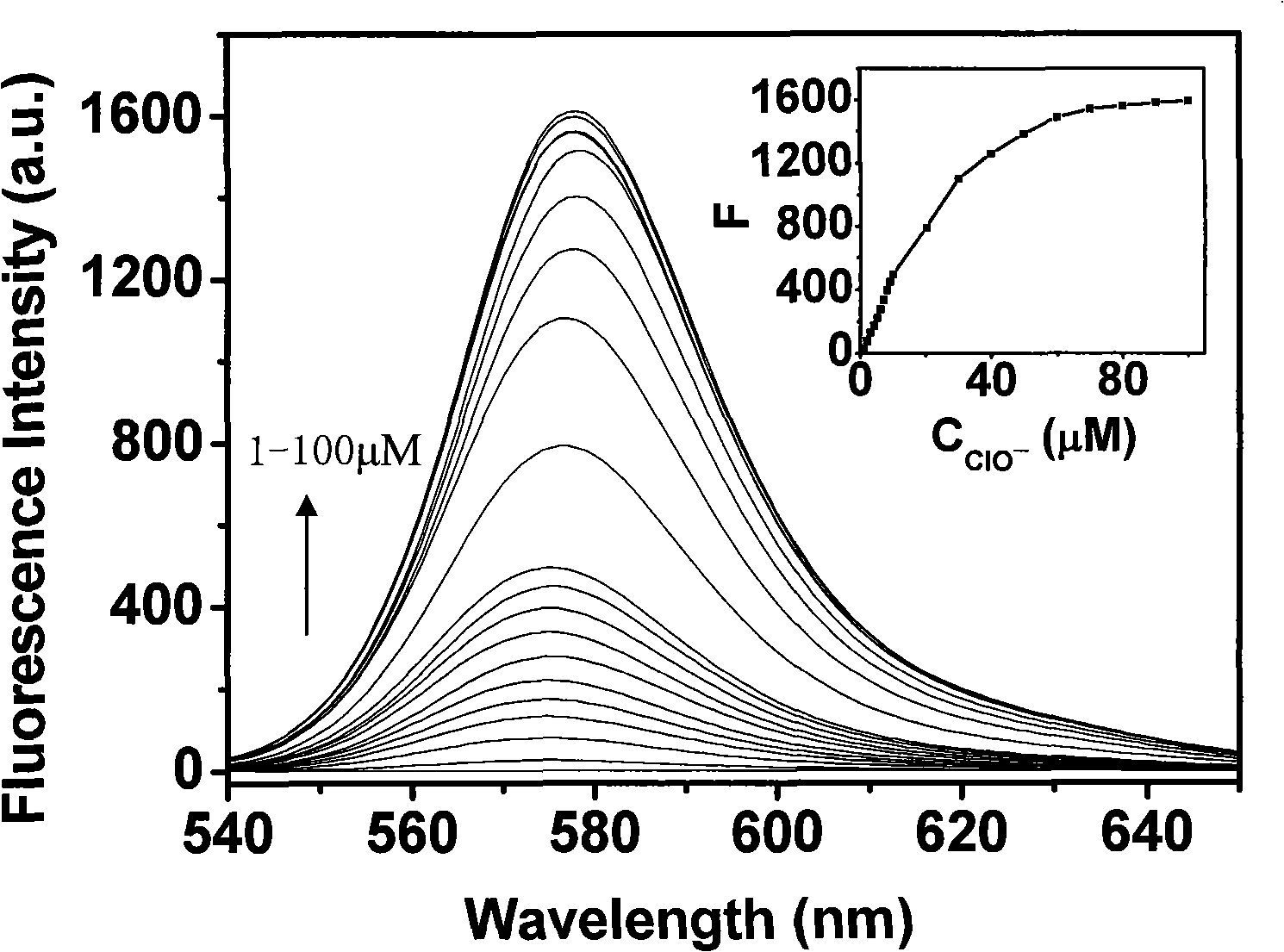
[0031] Add 100μL of the reagent—N-benzoylrhodamine B hydrazine mother solution (1mM THF solution) into a 10mL test tube, dilute to 3mL with THF, then add 2mL secondary deionized water and 3mL 0.1M Na 2 B 4 O 7 -NaOH buffer solution (pH=12.2), and finally add different amounts of sodium hypochlorite mother liquor and dilute the volume to 10 mL with secondary deionized water, shake and react for 30 minutes at room temperature, and measure the fluorescence spectrum of the reaction system.
[0032] image 3 Is the relationship between the concentration of sodium hypochlorite and the fluorescence spectrum of the reaction system (embedded figure: the linear relationship between the fluorescence intensity of the reaction system and the concentration of sodium hypochlorite, λ ex / em =520 / 578nm). Such as image 3 As shown, the excitation and emission wavelengths of the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com