Method for purifying lysostaphin by antibody affinity chromatography
A lysostaphin enzyme and a chromatographic purification technology are applied in the field of chromatographic purification of lysostaphinase, and can solve the problems of short column efficiency of affinity adsorption column, low adsorption capacity, and inability to be applied to industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
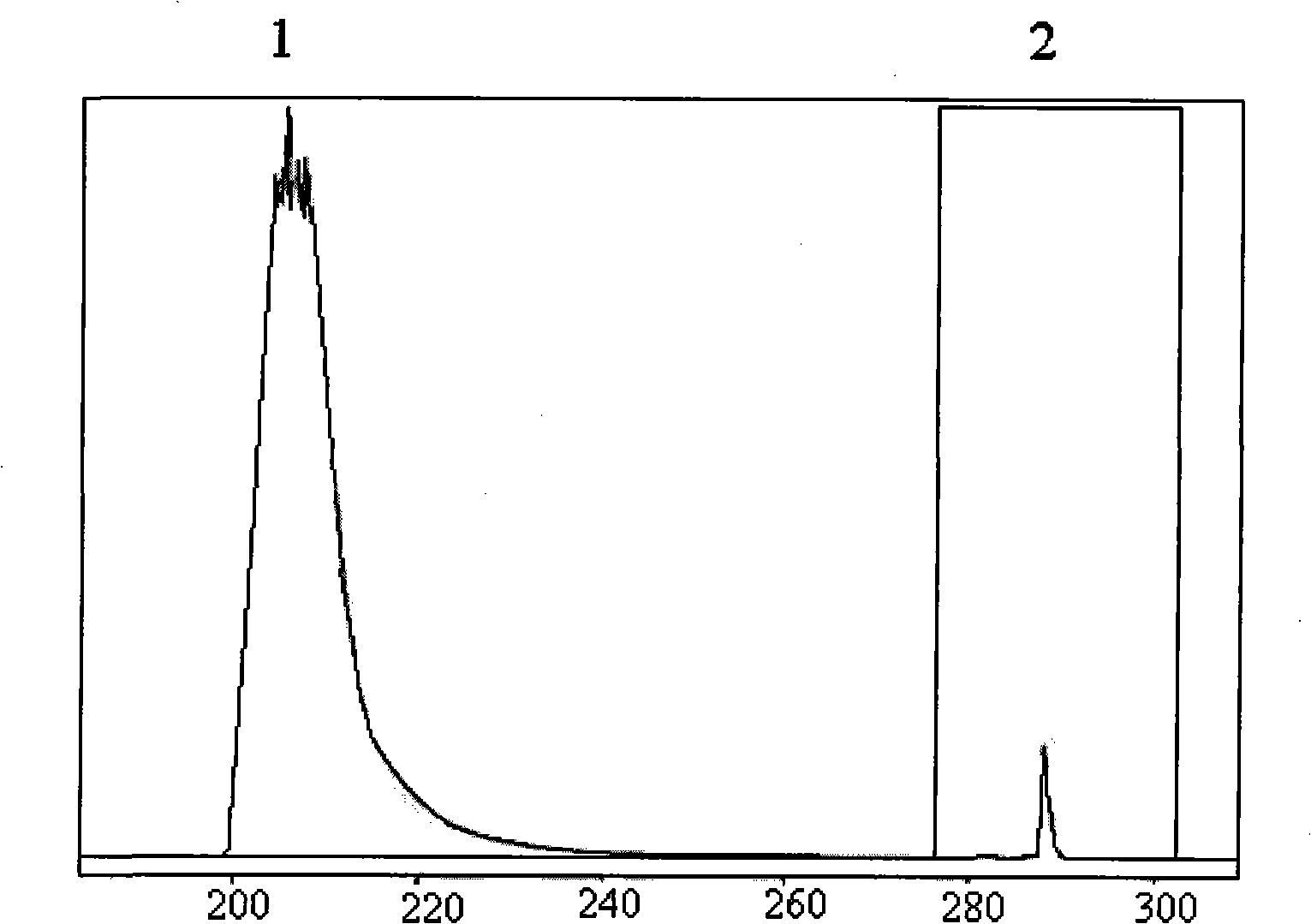
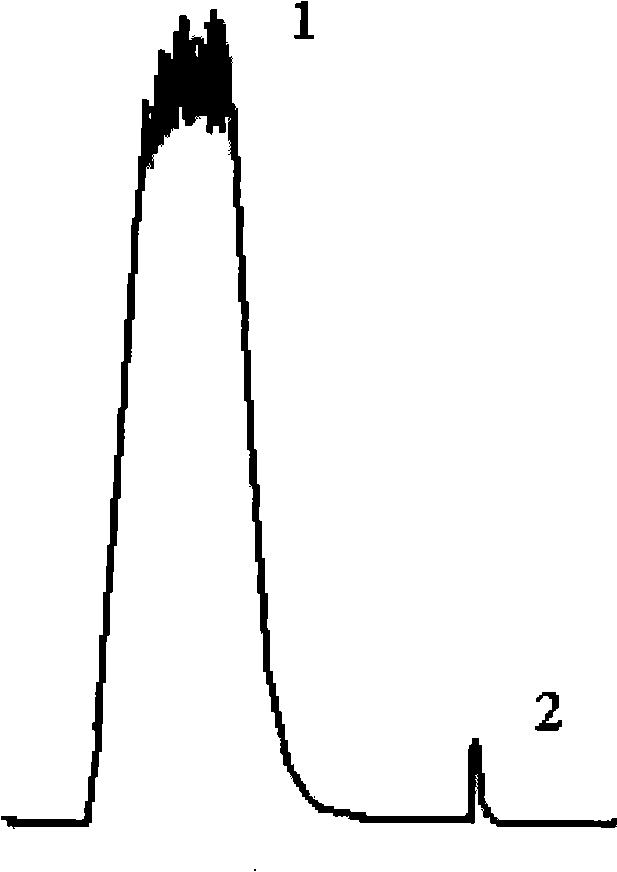
Image
Examples
Embodiment 1
[0048] Purification of monoclonal antibody to lysostaphin:
[0049] Chromatography medium: 5ml protein A hightrap prepacked column, purchased from Pharmacia Biotechnology Co., Ltd.
[0050] Monoclonal antibody sample: Using lysostaphin as an antigen, immunize BalB / c mice according to the conventional monoclonal antibody preparation method to prepare 15ml of mouse ascites containing lysostaphin monoclonal antibody. Wherein, the content of lysostaphin monoclonal antibody is 0.16mg / ml ;
[0051] Chromatography equipment: AKTA explorer 100 protein purification development system, purchased from Pharmacia Biotechnology Co., Ltd.
[0052] Equilibration and washing buffer: PBS buffer at pH 8.0.
[0053] Elution buffer: Gly-HCl buffer at pH 2.8 containing 0.1 mol / L glycine.
[0054] Equilibrium process: balance 5ml protein A prepacked column with PBS buffer solution with pH 8.0, pass it into the prepacked column at a flow rate of 3ml per minute, the amount is 6 times the volume o...
Embodiment 2
[0061] Purification of Lysostaphin Polyclonal Antibody:
[0062] Chromatography medium: 5ml protein A hightrap prepacked column, purchased from Pharmacia Biotechnology Co., Ltd.
[0063] Polyclonal antibody sample: Using lysostaphin as an antigen, New Zealand rabbits were immunized according to the conventional polyclonal antibody preparation method, and 8ml of rabbit antiserum containing lysostaphin polyclonal antibody was prepared. Wherein, the content of lysostaphin polyclonal antibody is 2.75mg / ml :
[0064] Chromatography equipment: AKTA explorer 100 protein purification development system, purchased from Pharmacia Biotechnology Co., Ltd.
[0065] Equilibrium and washing buffer: Tris-HCl buffer at pH 8.0 containing 0.1mol / LTris.
[0066] Elution buffer: Gly-HCl buffer at pH 2.8 containing 0.1 mol / L glycine.
[0067] Equilibrium process: equilibrate 5ml protein A prepacked column with Tris-HCl buffer solution containing 0.1mol / L Tris at pH 8.0, the flow rate is 4ml pe...
Embodiment 3
[0073] Purification of Lysostaphin by Affinity Chromatography
[0074] Affinity medium: a chromatographic column filled with 3 ml of Sepharose 4B gel (purchased from Pharmacia Biotechnology Co., Ltd., activated by CNBr) coupled with the monoclonal antibody prepared in Example 1.
[0075] Sample: 10ml of Escherichia coli culture supernatant containing lysostaphin gene prepared according to Chinese patent CN1900290, containing 964 units of lysostaphin, adding an equal volume of 0.1mol / L Tris-HCl at pH 8.0 containing 0.15mol / L NaCl Buffer, prepare 20ml of sample solution.
[0076] Chromatography equipment: AKTA explorer 100 protein purification development system, purchased from Pharmacia Biotechnology Co., Ltd.
[0077] Equilibrium and washing buffer: Tris-HCl buffer with pH 8.0 containing 0.1mol / L NaCl;
[0078] Elution buffer: pH 4.3 acetate buffer containing 0.1 mol / L sodium acetate.
[0079] Neutralization buffer: Tris-HCl buffer at pH 8.0 containing 1mol / LTris.
[0080]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
| Specific vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com