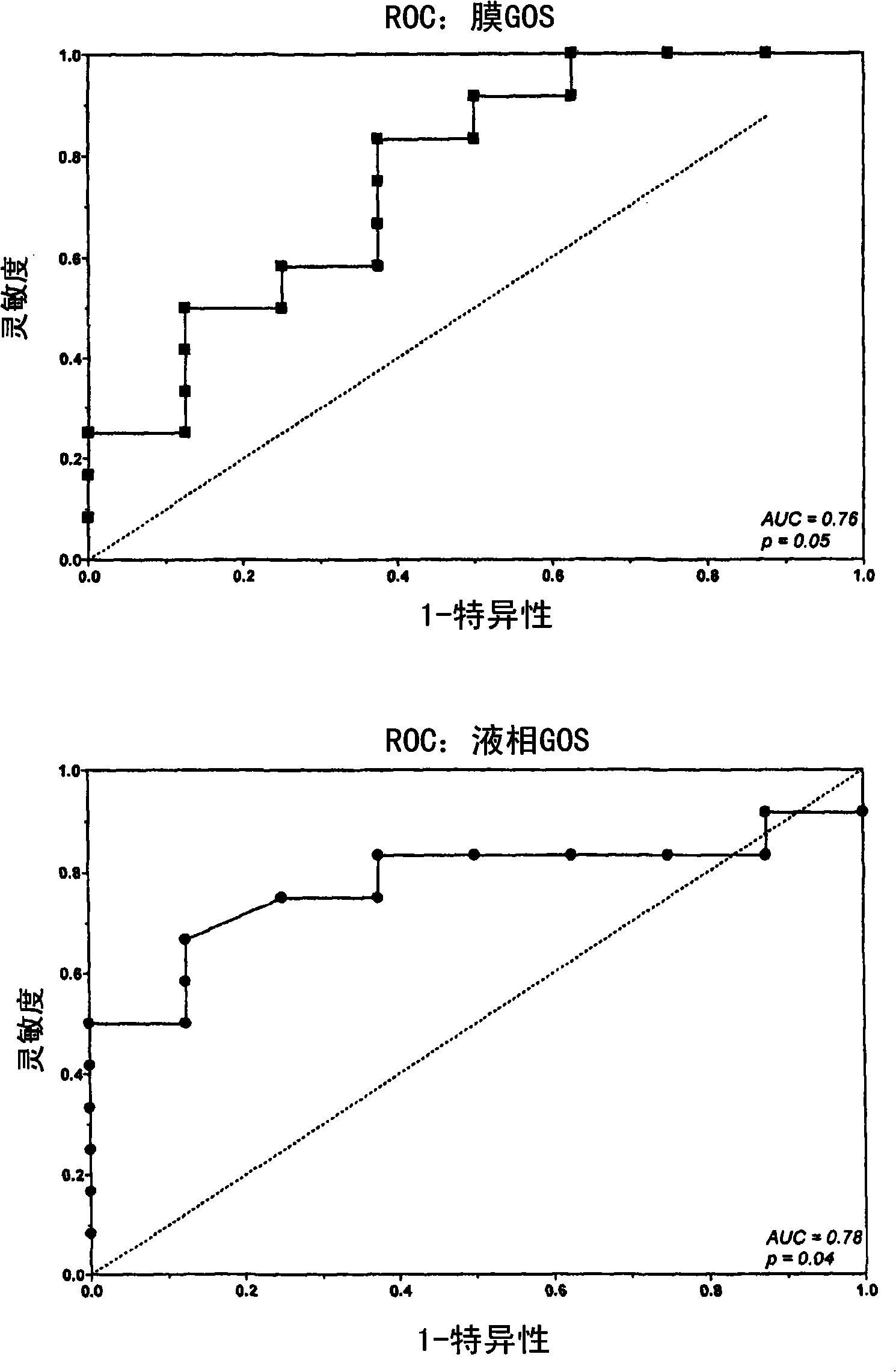
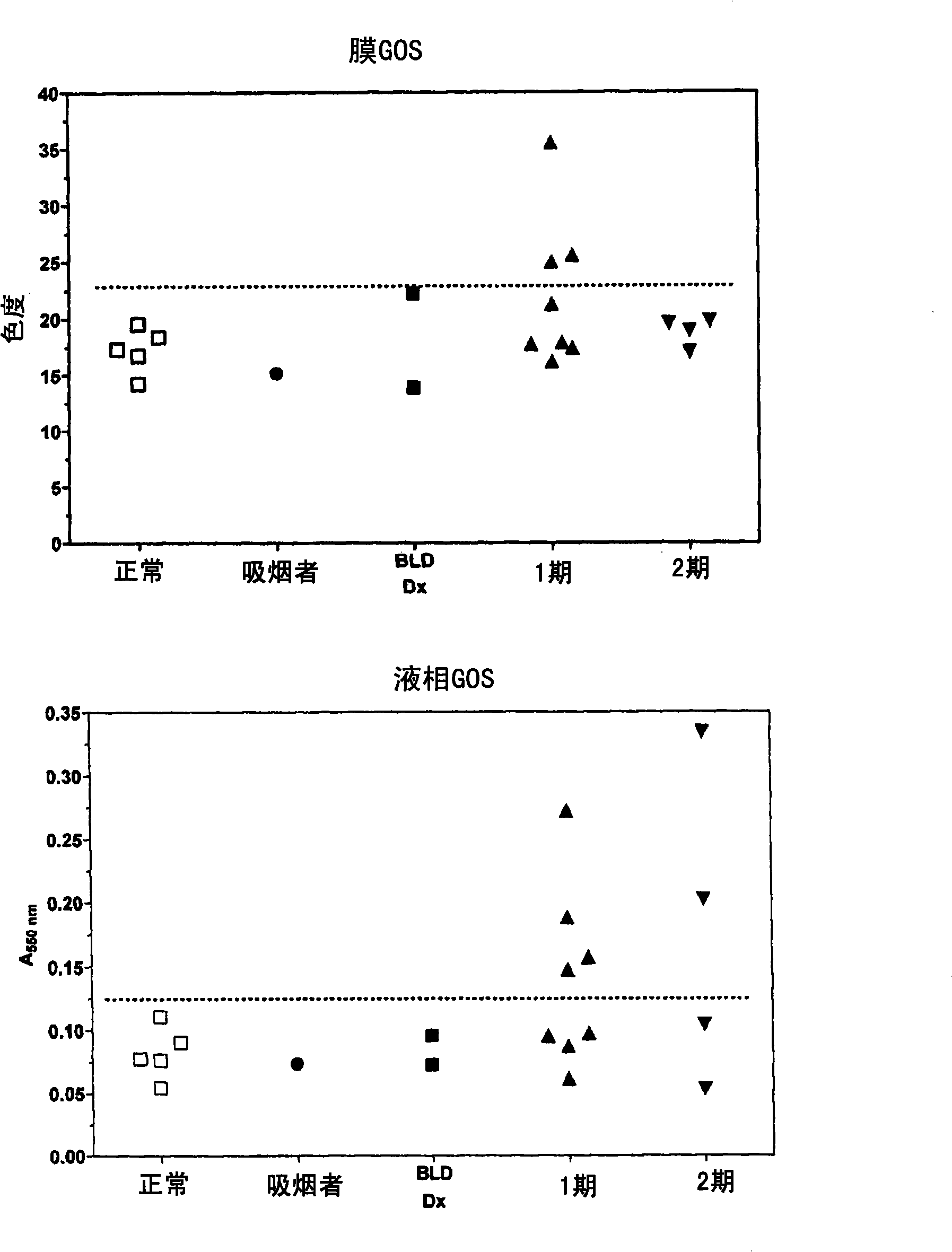
Liquid-phase galactose oxidase-schiff's assay
A technology of oxidants and sugars, applied in biochemical equipment and methods, microbiological determination/inspection, measuring devices, etc., can solve the problems of reducing the background of non-specific reaction products, eliminate the uncertainty of false negatives, and be clinically beneficial Effect of performance, report time reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Rectal Mucus
[0056] Rectal mucus was obtained by digital examination with a lubricated, gloved finger. In order to evaluate the reactivity of the above-mentioned mucus with GOS, it is first necessary to immobilize the mucus on a water-insoluble substrate (such as a membrane filter, glass slide). For liquid-phase GOS assays, mucus is recovered from the glove with the aid of a solubilizing agent, preferably in small amounts to minimize sample dilution. Alternatively, mucus samples can be collected onto swabs (cotton, polyester, polyamide, foam, alginate), extracted, and then tested with GOS in solution. Calcium alginate constituents are particularly suitable for sample recovery due to the solvation of the test fiber in some reagents (sodium citrate, glycerol phosphate, sodium polymetaphosphate, EGTA or EDTA) to form clear gels or solutions . Mucus released into the gel / solution can be determined with the following GOS:
[0057] 1) Incubate dissolved algin...
Embodiment 2
[0060] Example 2: Saliva and lung sputum
[0061] Saliva is collected in a cup, which is fluid sufficient for aspiration without solubilization or mucolytic agent pretreatment. Centrifuge prior to aspiration analysis to remove host buccal and bacterial cells from saliva. Saliva obtained from coughed up phlegm can be centrifuged. Saliva can be directly treated with GOS.
[0062] Sputum is a thick, gelatinous respiratory secretion that, in addition to normal and abnormal lung cells, contains mucin macromolecules (high molecular weight glycoproteins), bacterial polysaccharides and genetic material, host leukocyte DNA and actin filaments. Sputum is often coughed up with saliva and can be separated by centrifugation. Typically, sputum is collected after deep inspiration and vigorous coughing, but induction with hypertonic saline (eg, ≥3% NaCl) may be required. The consistency of sputum renders it immiscible with aqueous reagents. This precludes direct reaction with GOS unless...
Embodiment 3
[0069] Example 3: Nipple discharge
[0070] Nipple discharge (NAF) may be clear, slightly cloudy and / or discolored. It facilitates imbibition, so NAF can be tested directly with the GOS procedure without disulfide reducing agent pretreatment. In general, the method is essentially the same as for rectal mucus, saliva, and lung sputum, with adjustments for sample and reagent volumes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com