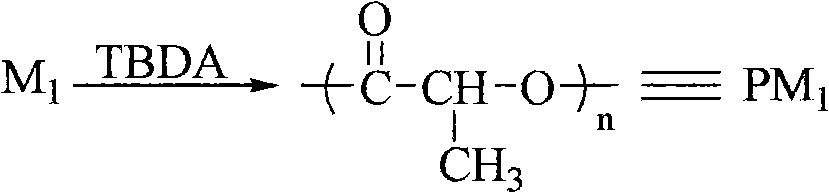Process for synthesizing acetate bicyclo guanidine and catalysis synthesis for poly-lactide and poly-serine morpholine diketone
The technology of a serine morpholinedione and a synthesis method, which is applied in the field of medical biodegradable materials, can solve problems such as hidden safety hazards and removal of tin-containing catalysts, and achieves the effects of low production cost, high product yield and high biosafety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
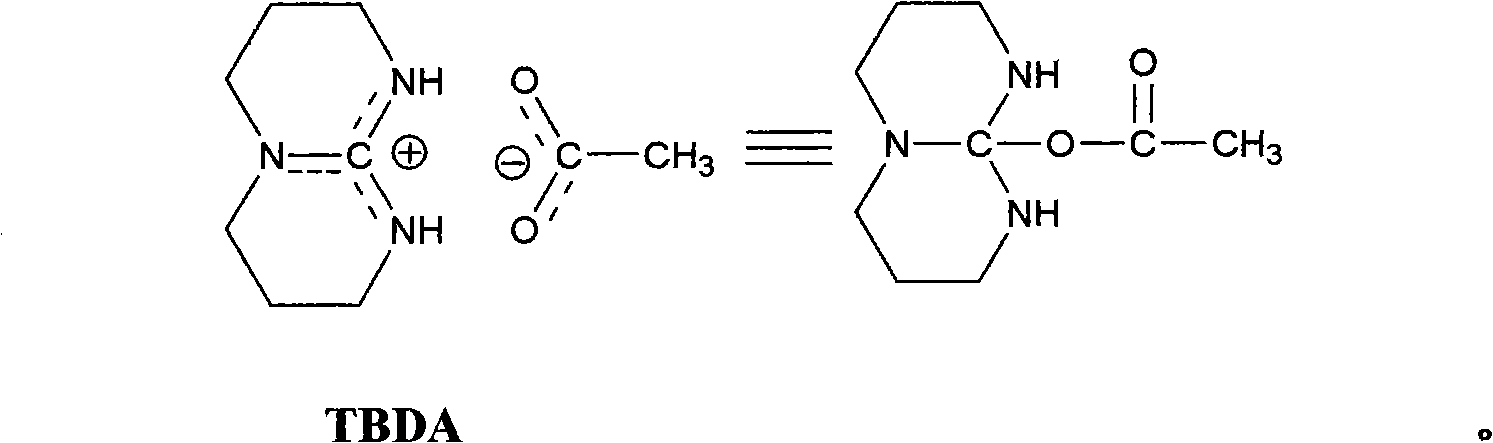
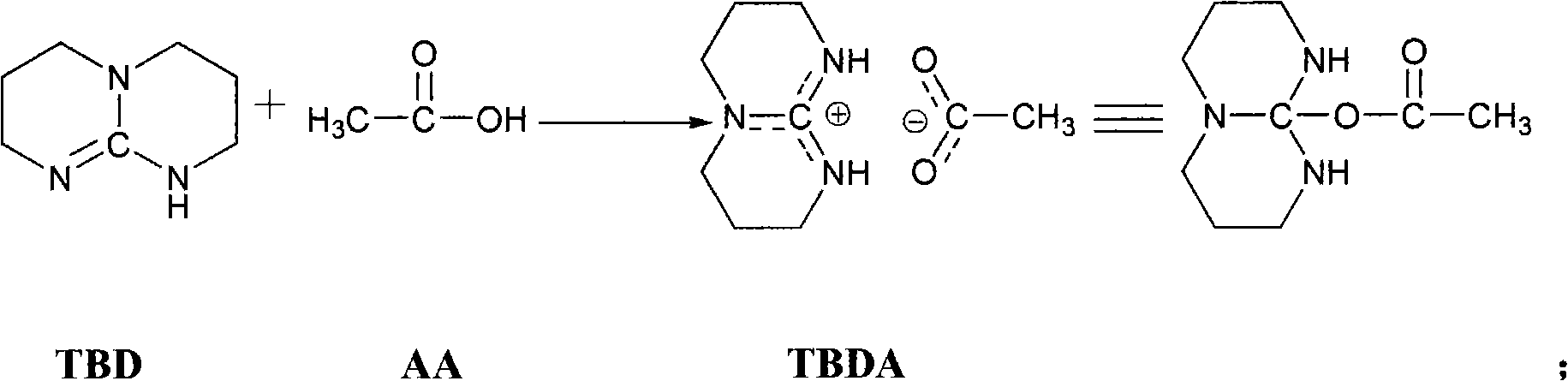
[0028] The preparation of embodiment 1 bicyclic guanidine acetate
[0029] Bicyclic guanidine (TBD) 0.5127g (3.69mmol) is placed in the reactor, after repeating vacuum-filling argon three times, add 3ml distilled water in the reactor under argon atmosphere, after the bicyclic guanidine dissolves completely under room temperature, add to Add 0.21 mL (3.69 mmol) of acetic acid into the reactor, and react at 80° C. for 9 hours. The reaction was stopped and cooled to room temperature, the water was distilled off under reduced pressure, and vacuum-dried for 24 hours to obtain bicyclic guanidine acetate as a white solid with a yield of 88.0% and a product purity of ≥98%.
Embodiment 2
[0030] The preparation of embodiment 2 bicyclic guanidine acetate
[0031] Bicyclic guanidine (TBD) 0.5026g (3.61mmol) is placed in reactor, after repeating vacuum-filling argon three times, under argon atmosphere, add 3ml distilled water in reactor, after the complete dissolving of bicyclic guanidine at room temperature, add to Add 0.20 mL (3.61 mmol) of acetic acid into the reactor, and react at 80° C. for 7 hours. The reaction was stopped and cooled to room temperature, the water was distilled off under reduced pressure, and vacuum-dried for 24 hours to obtain bicyclic guanidine acetate as a white solid with a yield of 90.5% and a product purity of ≥98%.
Embodiment 3
[0032] Example 3 Synthesis of poly-L-lactide catalyzed by bicyclic guanidine acetate (this example and the following reaction conditions for synthesizing polylactide are also applicable to polyserine morpholine dione, no further examples, the same below)
[0033] Weigh 1.000g (6.90mmol) of L-lactide and 0.0138g (0.0690mmol) of bicyclic guanidine acetate respectively and place them in a reaction kettle. React in the bath for 50min. After the reaction, dissolve the polymer with 3 mL of acetone, slowly drop it into distilled water to precipitate the polymer, remove the distilled water by filtration, and dry the obtained solid under vacuum at 40° C. for 24 hours. Yield: 87.09%, Mn=3.9×10 4 , PDI=1.15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com