Process for disassembling phosphate ore by mixed acid and coproducing potassium dihydrogen phosphate, hydrogen phosphate and combined fertilizer
A technology for co-production of potassium dihydrogen phosphate and calcium hydrogen phosphate, applied in chemical instruments and methods, sulfate/bisulfate preparation, phosphorus compounds, etc. The effect of dealing with the problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
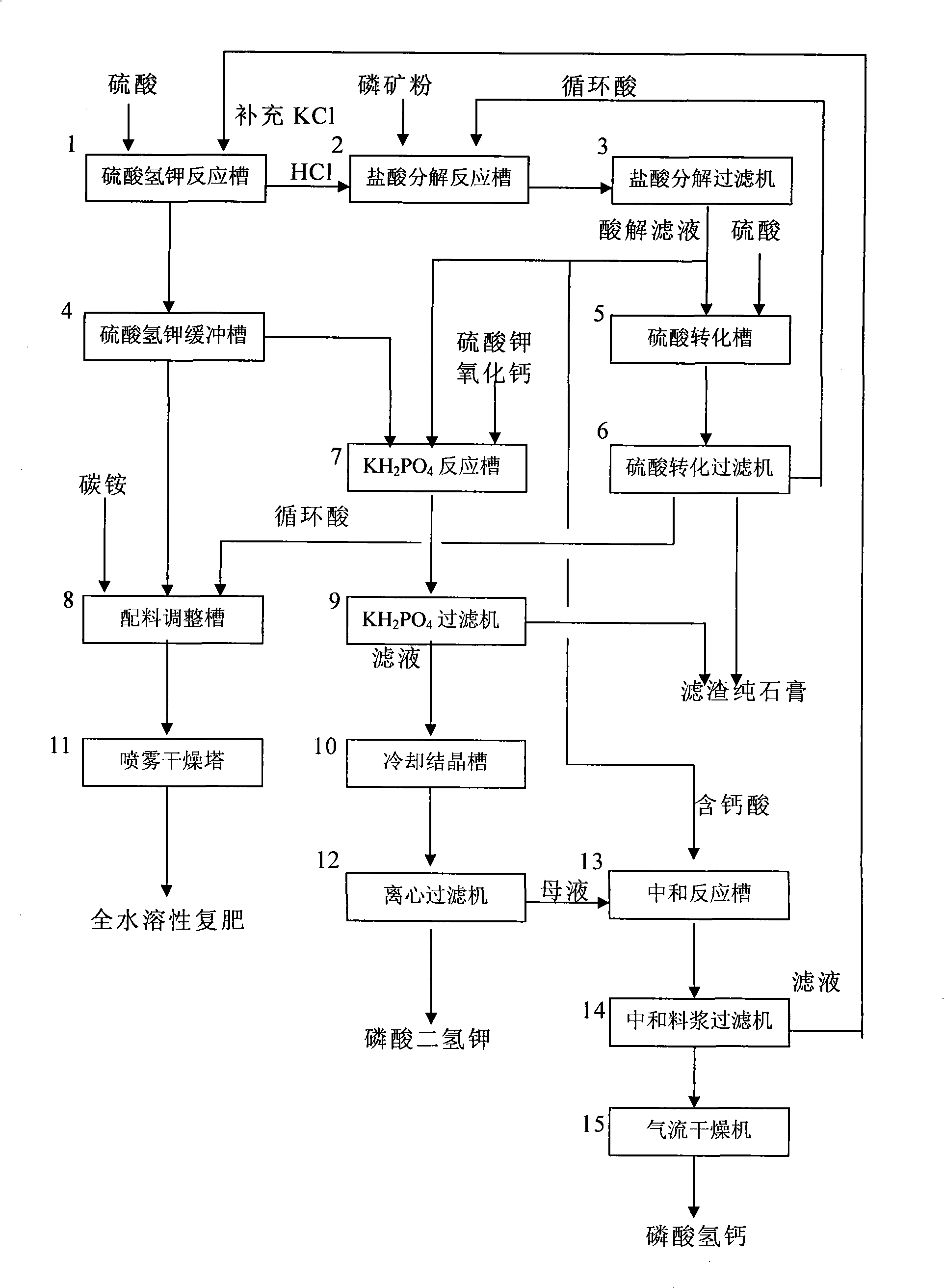
[0078] Embodiment 1: see Fig. 1.
[0079] Decomposing phosphate rock with mixed acid to produce calcium hydrogen phosphate and potassium dihydrogen phosphate comprises the following steps:
[0080] a, preparation of hydrogen chloride and potassium bisulfate:
[0081] In potassium bisulfate reaction tank 1, 16 tons of filtrate (KCl content 10%) after filtering calcium hydrogen phosphate in the input step e, add 11.9 tons of Repone K (the completion of batching contains 13.5 tons of Repone K in the slurry) , stir evenly and be prepared into a slurry containing 46% of potassium chloride. According to the mol ratio of sulfuric acid and Repone K is the ratio of 1: 1, in this potassium bisulfate reaction tank 1, add sulfuric acid (18.12 tons of sulfuric acid are added in total), stirring reaction. The reaction temperature is 60°C (without heating, the heat of reaction is achieved by itself).
[0082] The total amount of aqueous solution of potassium hydrogensulfate after the reac...
Embodiment 2
[0093] Embodiment 2: see Fig. 1.
[0094] A method for decomposing phosphate rock with mixed acid to co-produce potassium dihydrogen phosphate, calcium hydrogen phosphate and compound fertilizer, comprising the following steps:
[0095] a, preparation of hydrogen chloride and potassium bisulfate:
[0096] Get sulfuric acid and Repone K in the ratio of 1:1 by molar ratio; In potassium bisulfate reaction tank 1, water and Repone K are mixed and mixed with Repone K and the weight percentage composition that makes Repone is 40% slurry and stir Evenly, then add sulfuric acid, stir and react for 1 hour to obtain an aqueous solution of hydrogen chloride gas and potassium hydrogensulfate;
[0097] b. Phosphate rock absorbs hydrogen chloride for acidolysis reaction:
[0098] Get phosphate rock powder and water by the mass ratio of phosphate rock powder: water=1: 1, in hydrochloric acid decomposition reaction tank 2, phosphate rock powder and water are mixed and stirred into slurry, a...
Embodiment 3
[0114] Embodiment 3: see Fig. 1.
[0115] A method for decomposing phosphate rock with mixed acid to co-produce potassium dihydrogen phosphate, calcium hydrogen phosphate and compound fertilizer, comprising the following steps:
[0116] a, preparation of hydrogen chloride and potassium bisulfate:
[0117] Take sulfuric acid and Repone K in a ratio of 1:1 by molar ratio; in potassium bisulfate reaction tank 1, water and Repone Repone are mixed and prepared into a slurry that is 70% by weight of Repone Repone and stirs Evenly, then add sulfuric acid, stir and react for 4 hours to obtain an aqueous solution of hydrogen chloride gas and potassium bisulfate;
[0118] b. Phosphate rock absorbs hydrogen chloride for acidolysis reaction:
[0119] Get phosphate rock powder and water by the mass ratio of phosphate rock powder: water=1:3, in hydrochloric acid decomposition reaction tank 2, phosphate rock powder and water are mixed and stirred into slurry, absorb hydrogen chloride gas, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com