Use of total saponin from Panax notoginseng stalks and leaves in preparing immunoadjuvants
An immune adjuvant and total saponin technology, which is applied in the direction of medical preparations containing active ingredients, antibody medical ingredients, pharmaceutical formulas, etc., can solve the problems of short immunity period, aluminum glue preparation process, carcinogen hazards, etc., and achieve enhanced immunity Originality, extensive regulation, wide range of pharmacological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Determination of saponin content in Panax notoginseng stem and leaf total saponins
[0019] (1) Preparation of reference substance and test solution solution Precisely weigh 2.48 mg of ginsenoside Rb1 reference substance dried under reduced pressure at 60°C to constant weight in a 25mL measuring bottle, add methanol to dissolve and dilute to the mark, shake well, and prepare Each mL contains 0.992mg of the reference substance solution. Accurately weigh 25.6 mg of total saponins in stems and leaves of Panax notoginseng in a 25 mL volumetric flask, add methanol to dissolve and dilute to the mark, shake well, and prepare a test solution containing 1.024 mg per mL.
[0020](2) Examination of linear relationship Precisely draw 0.5, 1, 1.5, 2, 2.5, 3.0mL of reference substance solution, place them in 10mL stoppered test tubes respectively, evaporate the solvent, and add newly prepared 5% vanillin-glacial acetic acid Solution 0.2mL, perchloric acid 0.8mL, heated in ...
Embodiment 2
[0028] Example 2 Hemolytic Determination
[0029] Blood was collected from the rabbit heart with a vacuum blood collection tube, placed in a triangular flask filled with glass beads, shaken gently for 15 minutes to remove fibrin, added twice the amount of normal saline for washing, centrifuged at 2000r / min for 10 minutes, washed 3 times, red blood cells The suspension was diluted to 2% with physiological saline. Take the above-mentioned total saponins from the stems and leaves of Panax notoginseng, filter it with a 0.45 μm microporous membrane, and dilute it with normal saline to make a 0.25mg / mL-5mg / mL dilution, and set up four repetitions, and the different concentrations of the test product Take 2 mL of each solution, add 2 mL of 2% erythrocyte suspension in the test tube, mix well, and use normal saline and distilled water as the maximum and minimum hemolysis controls respectively. Place the above-mentioned test tubes in a constant temperature water bath at 37°C for 1 hou...
Embodiment 3
[0035] Example 3 In vitro lymphocyte transformation test
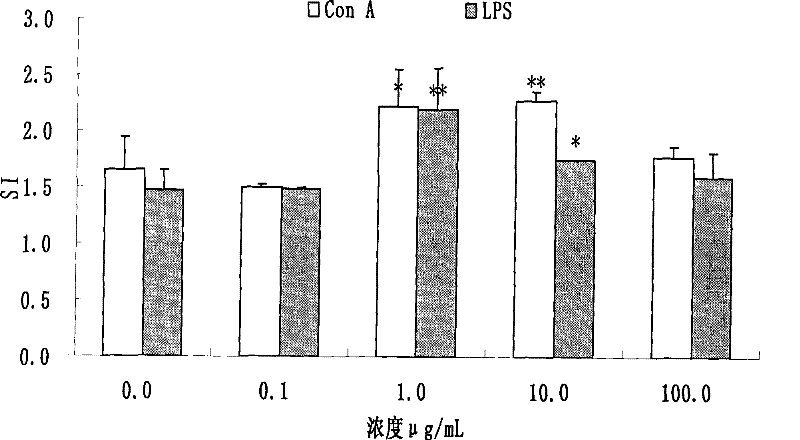
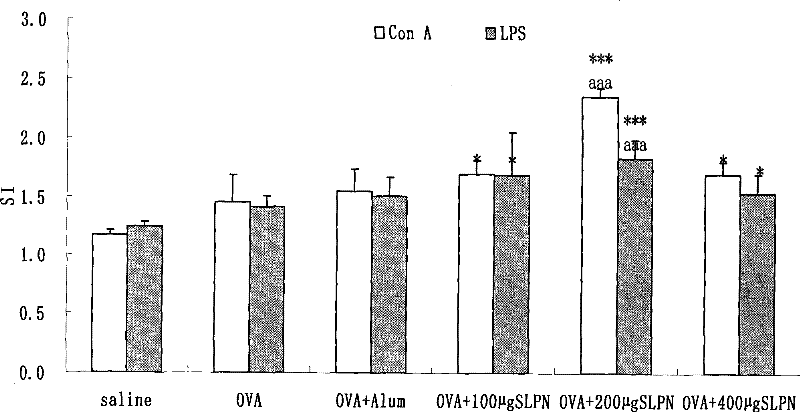
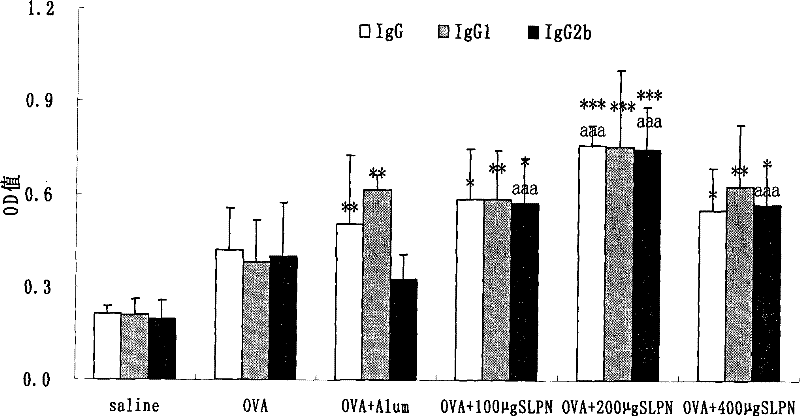
[0036] Take 100 μL splenocyte suspension (1x10 7 each sample / mL) in a 96-well plate, each sample has 12 wells, of which 4 wells are added with ConA (final concentration 5 μg / mL), 4 wells are added with LPS (final concentration 10 μg / mL), 4 wells are added with 100 μL RPMI1640 solution, each well Add different concentrations of total saponin dilutions of Panax notoginseng stems and leaves (final concentrations are 0 μg / mL, 0.1 μg / mL, 1 μg / mL, 10 μg / mL, 100 μg / mL), and incubate for 48 hours at 37°C and 5% CO2. 4 hours before the end, 50 μL / well of MTT (2 mg / mL) was added to each well, and culture was continued for 4 hours. Discard the liquid in each well, add 150 μL of dimethyl sulfoxide to each well, place in a dark place at room temperature for 15 minutes, measure the OD value with a microplate reader at a wavelength of 578 nm, and see the results in figure 1 .
[0037] Depend on figure 1 It can be seen that the to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com