Method for preparing 1-o-hydroxyphenyl butanone
A technology of o-hydroxyphenyl butanone and anisole, which is applied in the field of preparation of 1-o-hydroxyphenyl butanone, can solve the problems of large catalyst consumption, low total reaction yield, low product purity, etc., and achieve product The effect of high yield, short process flow and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
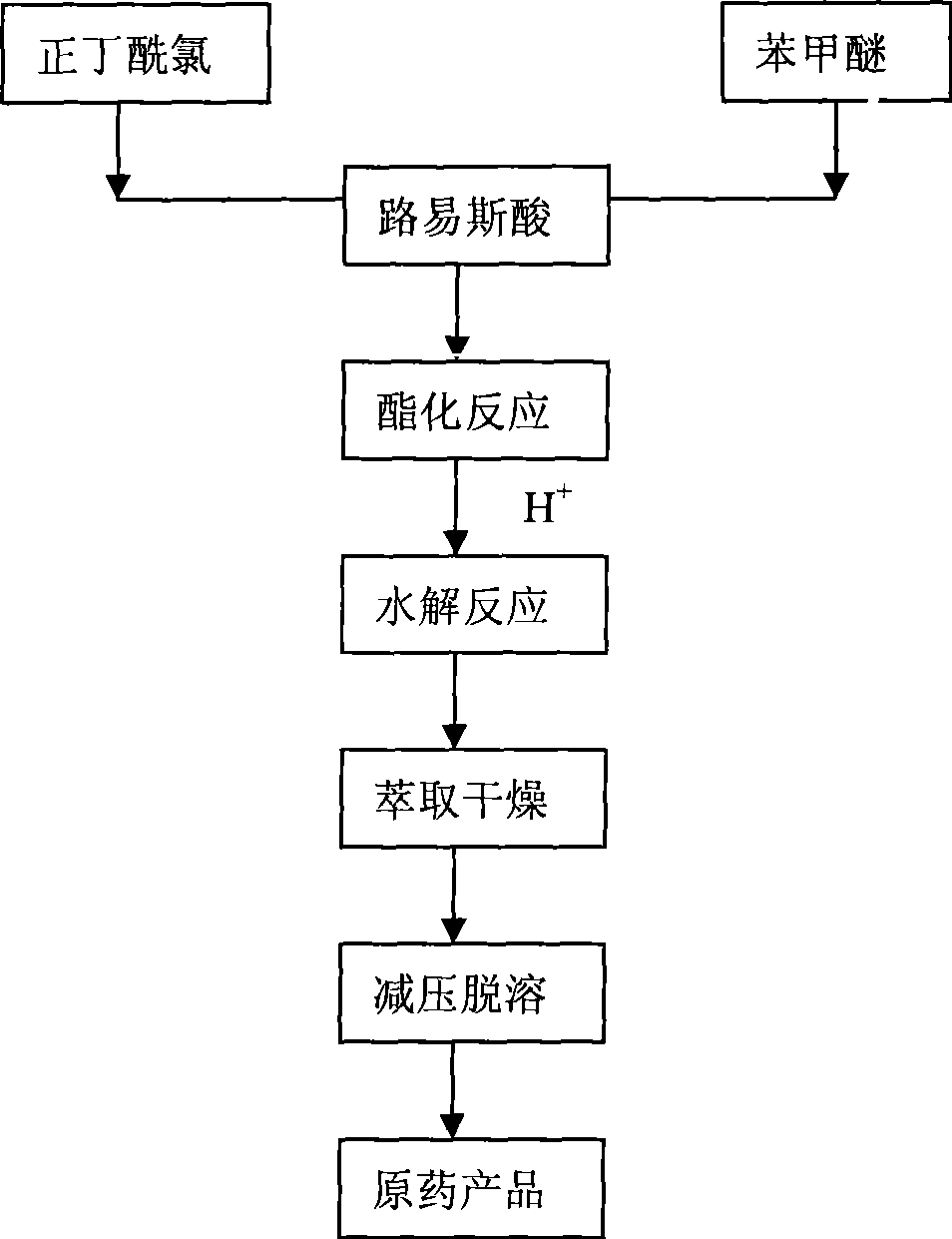
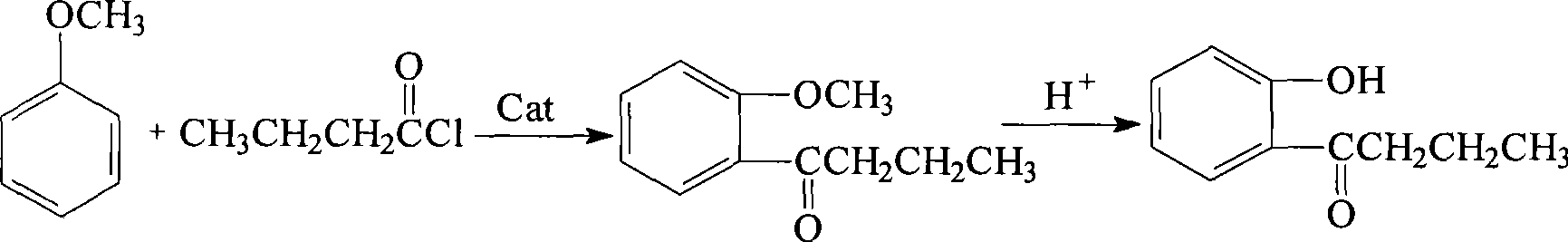
[0016] Add 54.5g (0.4moL) of 98% anhydrous aluminum trichloride and 80mL dichloroethane into a dry 500mL four-neck flask equipped with a stirrer, reflux condenser, and thermometer, and control the system temperature at about -5°C 22g (0.2moL) of 99% anisole was added dropwise to the reaction solution with stirring, and the temperature was controlled not to exceed 0°C; 21.8g (0.2moL) of 98% n-butyryl chloride was dissolved in 80mL of dichloroethane , after the addition of anisole is completed, then slowly add the dichloroethane solution of n-butyryl chloride dropwise to the reaction system for about 1 hour; Stir the reaction for 3 hours; after the reaction is completed, cool the system to room temperature, pour the reaction liquid into 130 g of crushed ice and concentrated hydrochloric acid mixture containing 10% hydrochloric acid by mass, let it stand for layers, separate the aqueous phase and the organic phase, and use 60 mL of the aqueous phase Dichloroethane was extracted t...
Embodiment 2
[0018] Add 99.5g (0.6moL) of 98% anhydrous iron trichloride, 150mL ethyl acetate in a dry 500mL four-neck flask with agitator, reflux condenser and thermometer, control the system temperature at about 0°C, Add 22 g (0.2 moL) of 99% anisole dropwise to the reaction solution under stirring, and control the temperature not to exceed 0°C due to exothermic reaction; dissolve 26.2 g (0.24 moL) of 98% n-butyryl chloride in 80 mL of ethyl acetate In the ester, after the anisole is added dropwise, slowly add the n-butyryl chloride solution dropwise to the reaction system, and the dropping time is about 1 hour; After 2 hours, the reaction was completed and the system was cooled to room temperature, and the reaction solution was poured into 230 g of crushed ice and concentrated hydrochloric acid mixture containing about 10% hydrochloric acid by mass, allowed to stand for layers, and the water phase and the organic phase were separated, and the water phase was mixed with 80 mL of acetic ac...
Embodiment 3
[0020] Add 111g (0.8moL) of 98% anhydrous zinc chloride and 150mL chlorobenzene in a dry four-necked flask with a stirrer, reflux condenser, and 500mL thermometer, and control the system temperature at about -4°C. 22g (0.2moL) of 99% anisole was added dropwise to the reaction liquid under the condition, due to the exothermic reaction, the temperature was controlled not to exceed 0°C; 24g (0.22moL) of 98% n-butyryl chloride was dissolved in 60mL of chlorobenzene, and After the anisole was added dropwise, the above-mentioned n-butyryl chloride solution was slowly added dropwise to the reaction system for about 1 hour. After the n-butyryl chloride dilution was added dropwise, the temperature was raised to reflux temperature, and the reaction was continued for 2.5 hours with stirring. After the reaction, the system was cooled to room temperature, and the reaction solution was poured into 180 g of crushed ice and concentrated hydrochloric acid mixture containing about 10% hydrochlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com