Modified glucan derivative and molded object thereof
A technology of dextran and derivatives, applied in the direction of polarizing elements, etc., can solve problems such as low glass transition temperature, insufficient heat resistance, and damage to the good properties of cellulose acetate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0125] (Preparation method of modified dextran derivative)
[0126] The modified dextran derivative of the present invention (first invention) can be obtained by reacting the dextran derivative with the aforementioned hydroxy acid component (ring-opening polymerization reaction or condensation reaction). That is, a modified dextran derivative can be produced by graft-polymerizing a hydroxy acid component on a dextran derivative. Moreover, when using a cyclic ester (such as lactide, etc.) ), and when a hydroxy acid (hydroxycaproic acid, etc.) is used as the hydroxy acid component, the grafting reaction is a condensation reaction (condensation grafting reaction). In the present invention, a ring-opening grafting reaction using a cyclic ester is generally suitably employed.
[0127] Moreover, the moisture content of the dextran derivative and the hydroxy acid component used in the graft polymerization (especially the ring-opening polymerization using a cyclic ester) is preferab...
Embodiment
[0300] Hereinafter, the present invention will be described in more detail based on examples, but the present invention is not limited by these examples. Moreover, in the examples, unless otherwise mentioned, "parts" means "parts by weight".
[0301] Also, in Examples, various properties were measured as follows.
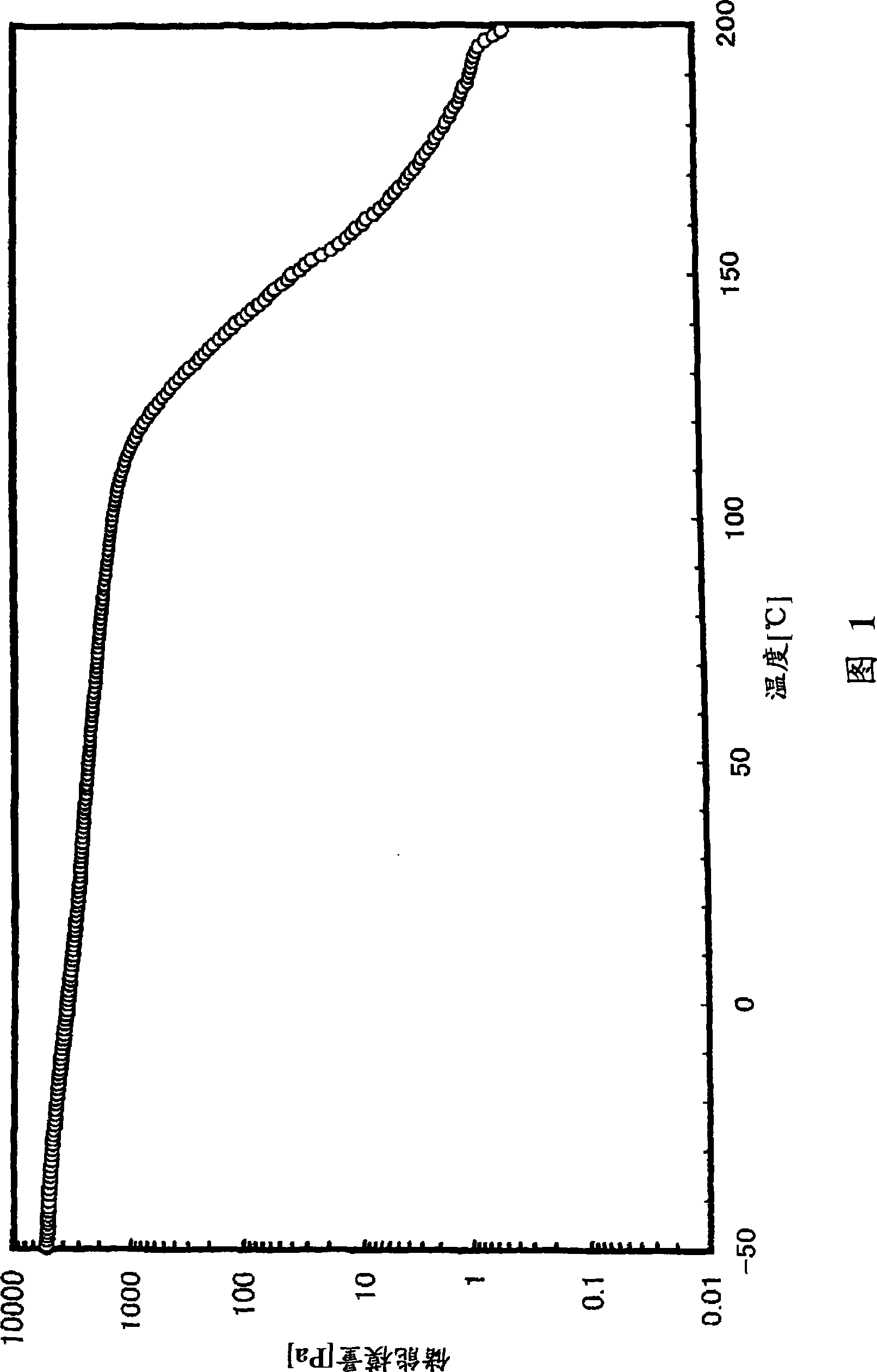
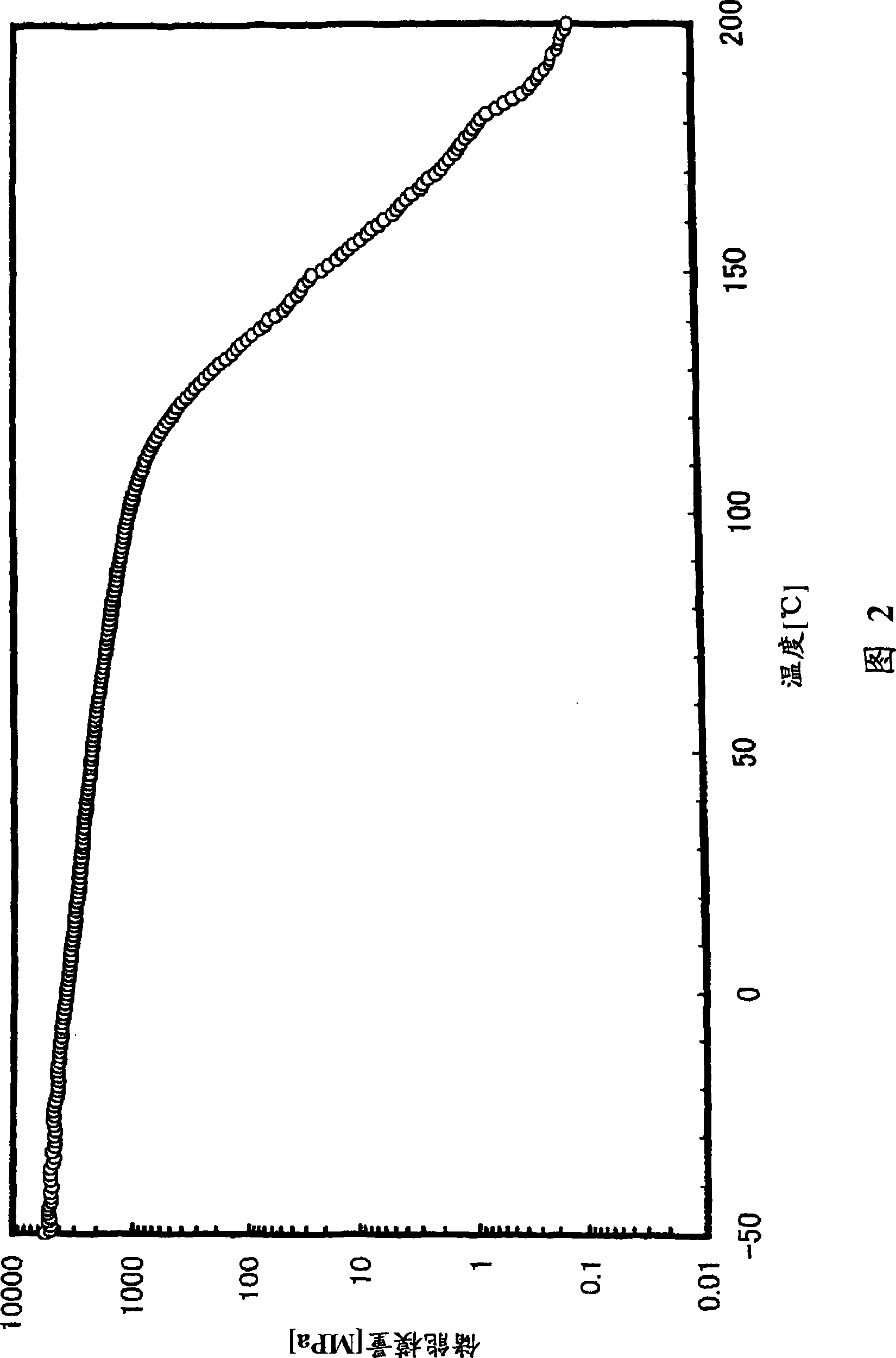
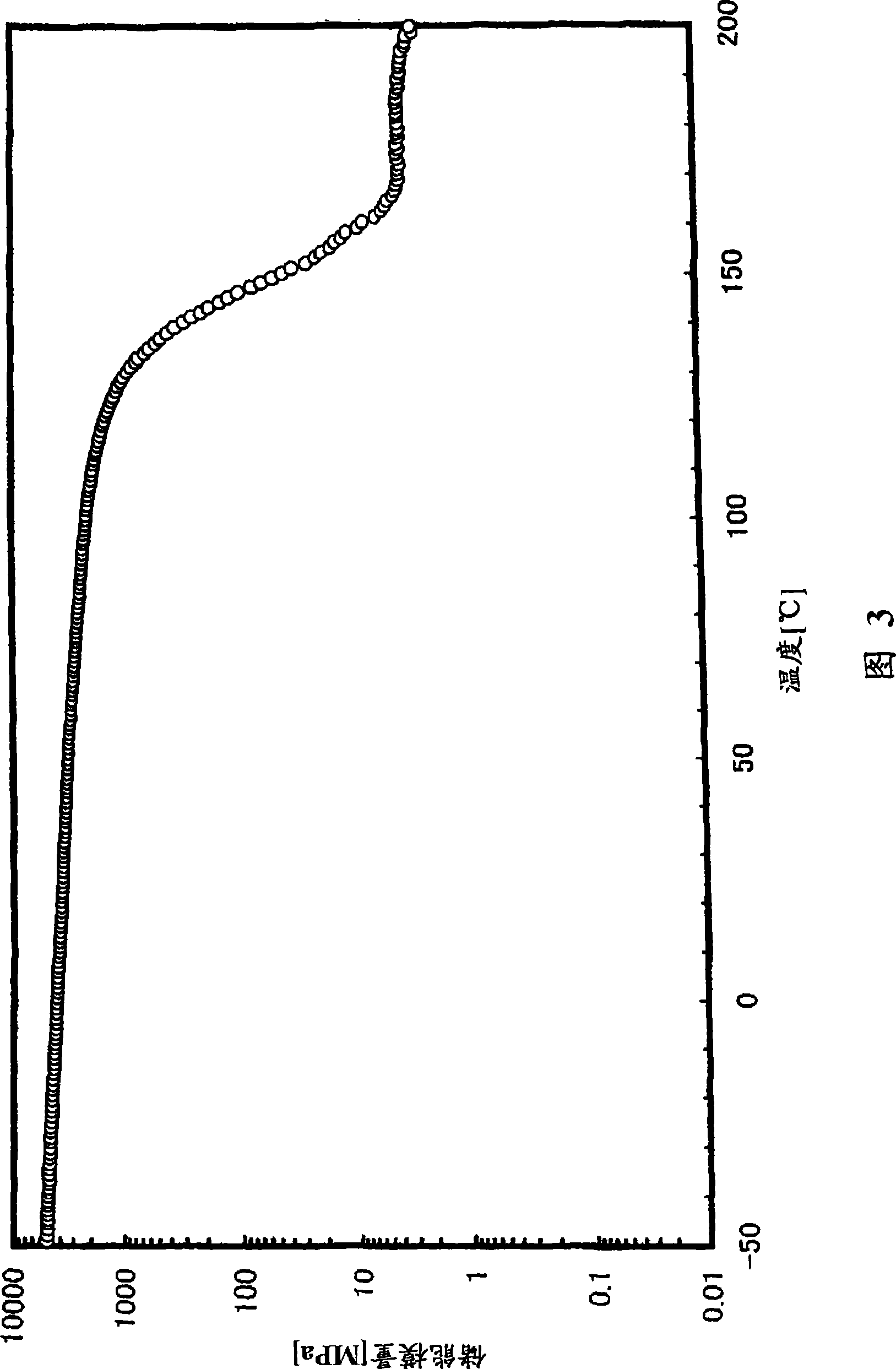
[0302] (glass transition temperature)
[0303] The glass transition temperature (Tg) was measured on the following conditions in accordance with the method of JIS K7121 using a high-sensitivity differential scanning calorimeter (manufactured by SEIKO Instruments Co., Ltd., "DSC6200").
[0304] Sample weight: 8.0mg
[0305] Nitrogen inflow: 40ml / min
[0306] Heating speed: 20°C / min
[0307] Cooling rate: 20°C / min
[0308] Measurement start temperature: 20°C
[0309] Determination termination temperature: 210°C
[0310]In addition, the glass transition temperature was measured in the same environment after placing the sample in a constant temperature and consta...
Embodiment A-1
[0333] (Synthesis of grafts)
[0334] Add 80 parts of cellulose acetate (manufactured by Daicel Chemical Industry Co., Ltd., L-20, degree of substitution: 2.41) into a reactor equipped with a stirrer and an anchor-type (いかり-type) stirring blade, and dry under reduced pressure at 110° C. and 4 Torr 4 hours. Then clean it with dry nitrogen, install a reflux cooling pipe, add 20 parts of ε-caprolactone and 67 parts of cyclohexanone (ANON) that have been dried and distilled in advance, heat to 160°C, and stir. Dissolve the cellulose acetate evenly. 0.25 part of monobutyltin trioctoate was added to this reaction liquid, and it heated at 160 degreeC for 2 hours with stirring. Then, the reaction liquid was cooled to room temperature, and the reaction was terminated to obtain a reaction product. Next, 10 parts of the reaction product were dissolved in 90 parts of chloroform, and then slowly added dropwise to 900 parts of methanol in a sufficient excess, and the precipitate (graft) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com