Fire resistant xylanase XynA1, gene for encoding the enzyme and uses thereof
A technology of xylanase and encoding gene, applied in the field of xylanase, can solve problems such as no literature reports, and achieve the effects of good thermal stability and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
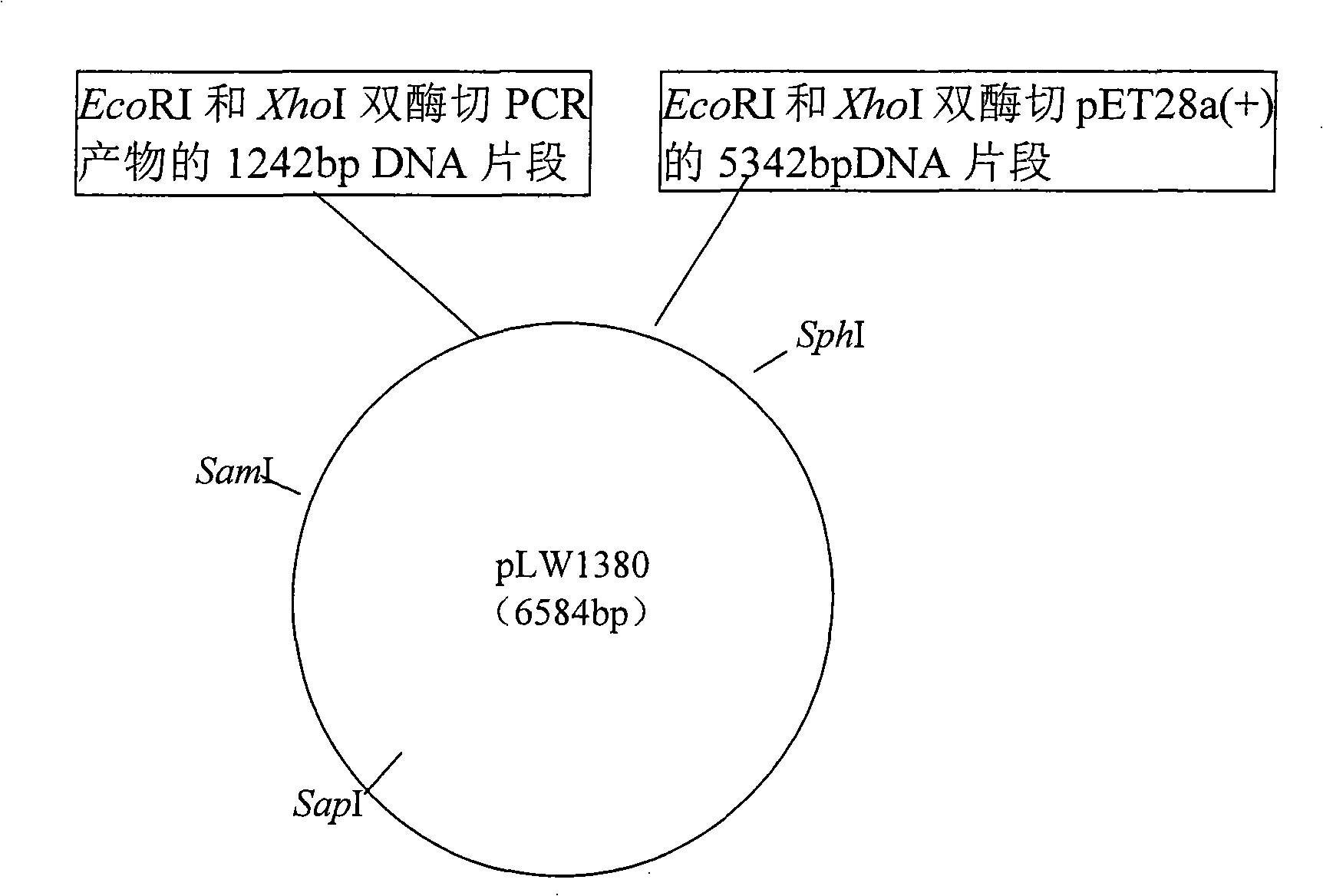
[0045] 1. Extraction of total DNA from Bacillus denitrophilus NG80-2 (CGMCC No.1228)
[0046] The extraction of total DNA from Geobacillus thermodenitrificans NG80-2 (CGMCC No.1228) proved that the gene encoding alcohol dehydrogenase can be isolated from the genome of Geobacillus thermodenitrificans NG80-2. Therefore, in this embodiment, the thermophilic denitrophilic Bacillus NG80-2 obtained from the oil well formation water separation of Guan 69-8 block, Dagang Oilfield, Tianjin, China (it is preserved in the General Microorganism Center of China Microbiological Culture Collection Management Committee) is used. The number is CGMCC No.1228, and the preservation date is October 9, 2004. It has applied for a domestic invention patent and obtained authorization. 3ml of the fresh culture cultured overnight was collected by centrifugation, and the bacteria were suspended in 250μl 50mM Tris buffer (pH8.0), and 10μl 0.4M EDTA (pH8.0) was added, mixed well, incubated at 37℃ for 20min...
Embodiment 2
[0062] Expression, purification and characterization of recombinant xylanase:
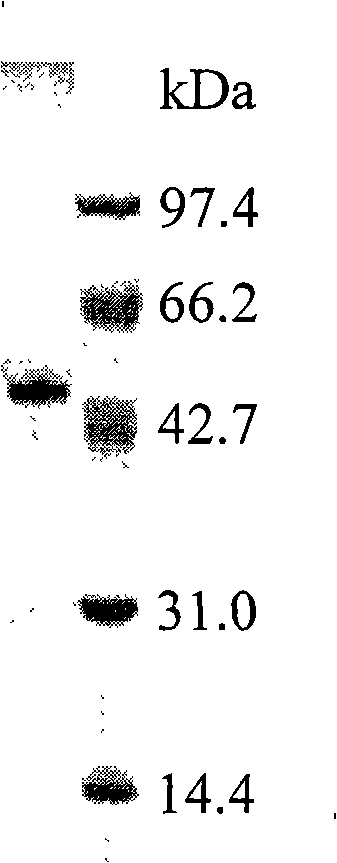
[0063] Insert the above-mentioned recombinant bacteria H1739 monoclonal into 20ml of LB medium containing 50μg / ml Kan, culture at 37°C and 180rpm for 12 hours, and then insert the culture into 200ml containing 50μg / ml Kan's LB medium, 37 ° C, 220 rpm to OD 600 When it is 0.6, add IPTG to a final concentration of 0.05mM, and induce at 45°C for 3 hours. The cells were collected by centrifugation at 5000rpm for 5min, suspended in 50mM Tris-HCl (pH8.0) buffer, disrupted by ultrasonic waves, centrifuged at 14000g for 20min, and the supernatant was the crude extract of alcohol dehydrogenase. This supernatant was purified by chelating agarose gel (Chelating Sepharose) nickel affinity column chromatography, and the enzyme preparation obtained showed a band on SDS-PAGE (see image 3 ). The basic properties of this enzyme system were determined using known standard methods of protein chemistry. The molec...
Embodiment 3
[0065] 1. Measure the specific activity of xylanase of the present invention when it acts on xylan substrates from different sources:
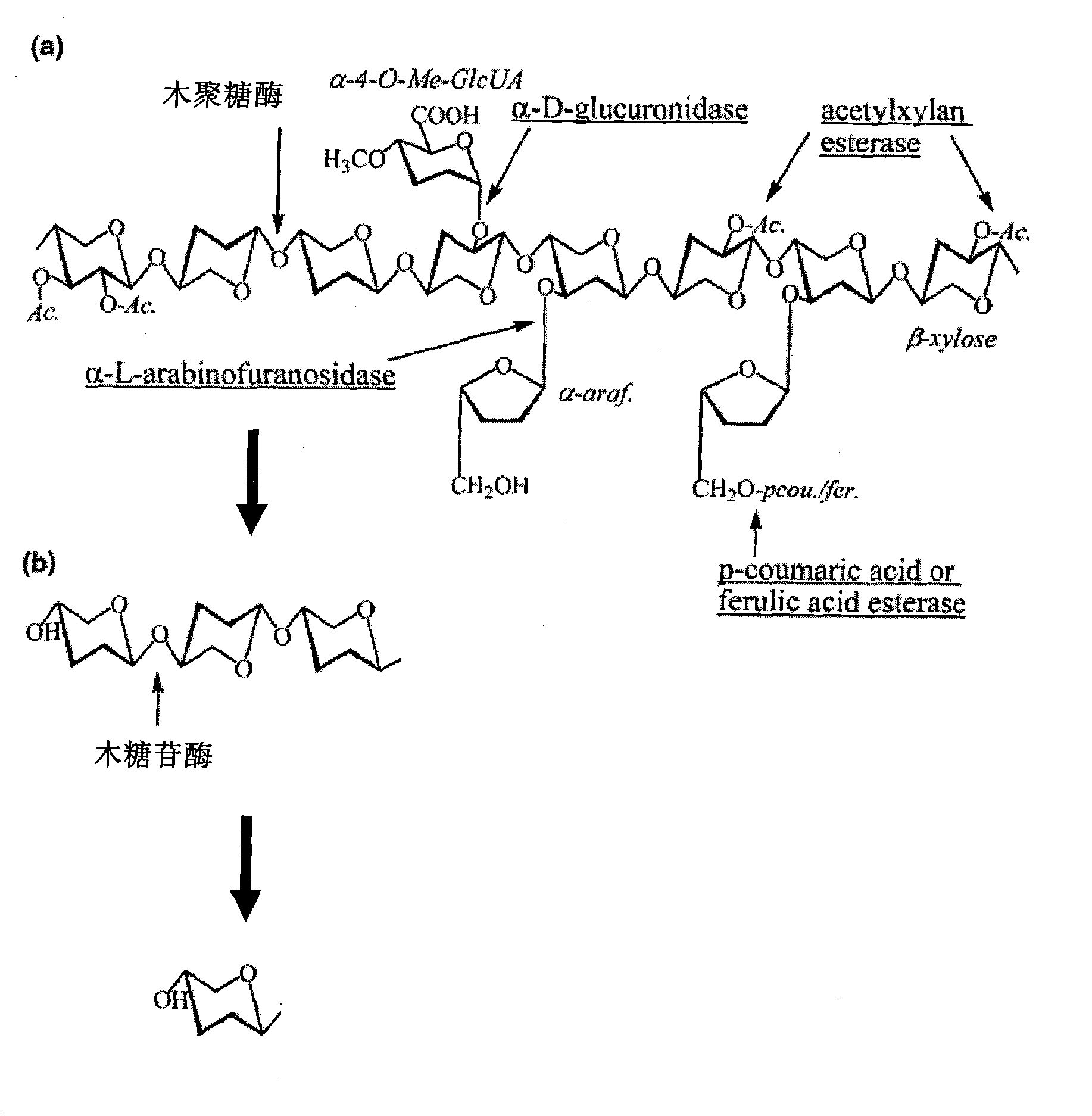
[0066] When xylanase is added to animal feed or paper pulp, the impact of its activity and specificity on xylan from different sources should be considered. Rye) xylan model substrates, beech xylan and birch xylan were used as wood-derived xylan model substrates to investigate their specific activities to xylans from different sources.
[0067] The xylanase prepared in the above-mentioned Example 2 can degrade xylan from different sources. This experiment is for three kinds of xylan substrates currently available for purchase: oat xylan (Sigma product number is XO627), beech wood Glycans (Sigma product number X4252) and birch xylan (Sigma product number X0502) were tested for enzyme activity. The specific method is: prepare the xylanase XynA1 reaction system (100 μl) as follows: add oat xylan, beech xylan, birch xylan substrate to a final con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com