Creatine sustained-release preparation and preparation process thereof medication
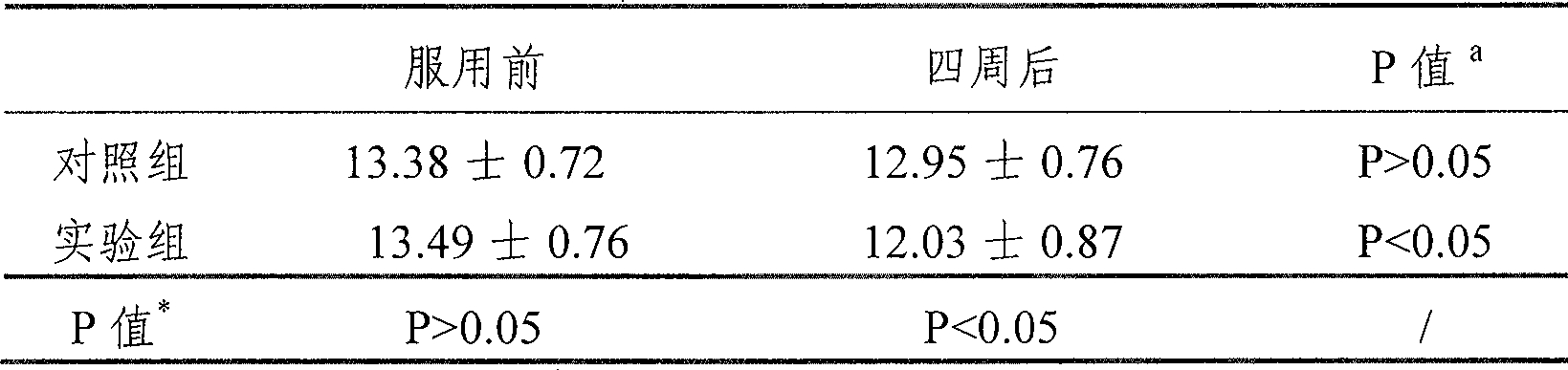
A technology of sustained-release preparations and creatine, applied in the field of creatine sustained-release preparations and its preparation, and creatine products, which can solve the problems of short half-life, increased burden on the kidneys, and waste, so as to promote muscle growth and avoid increased burden on the liver and kidneys , Improve the effect of absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 tablet
[0035] Weigh 12kg of creatine monohydrate, 8.5kg of hydroxypropylmethylcellulose, 1kg of methylcellulose, 1.75kg of lactose and 0.15kg of microcrystalline cellulose, and mix them in a mixer.
[0036] An appropriate amount of 20% ethanol solution is added to the above powder and granulated, the wet material is dried, and properly sieved to obtain dry granules.
[0037] The above-mentioned dry granules are mixed with 0.15 kg of magnesium stearate, and compressed in a tablet machine. Each tablet weighs 800mg.
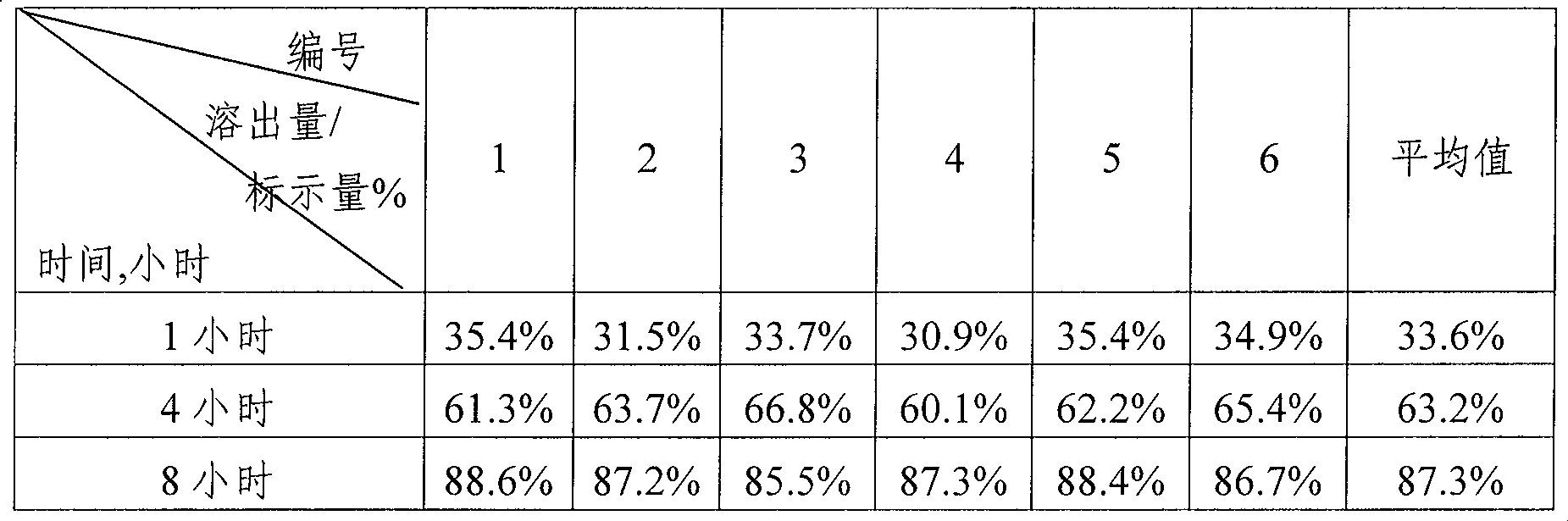
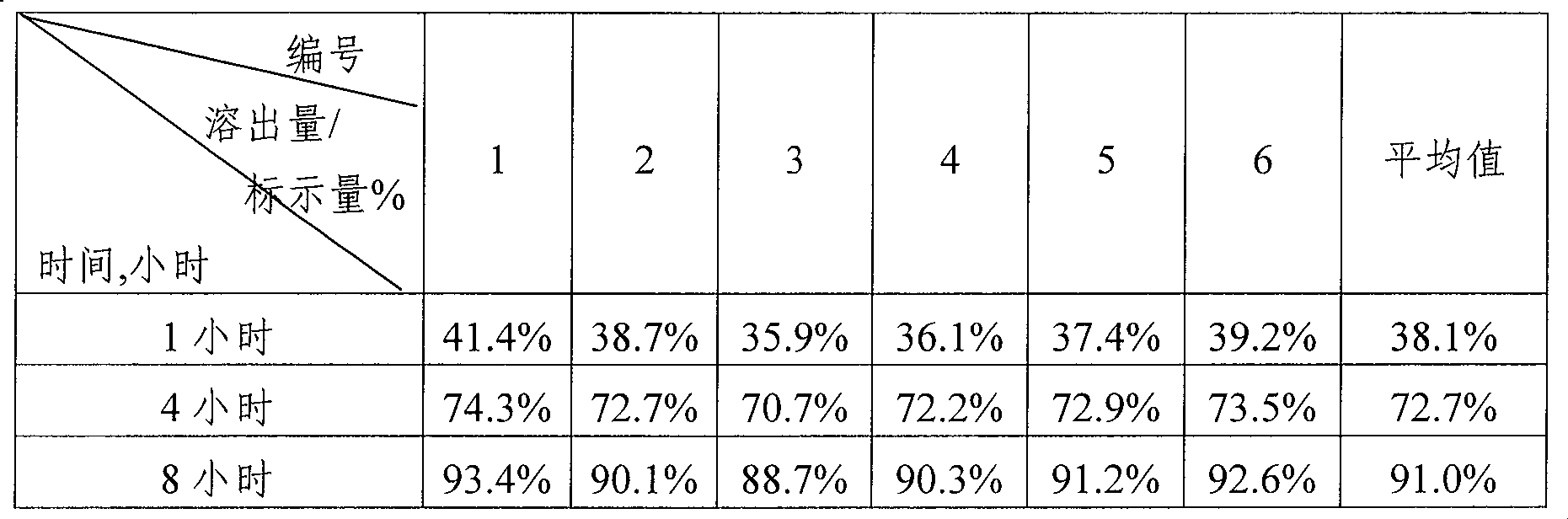
[0038] The obtained sustained-release creatine tablet has a percentage of creatine monohydrate of 51%, and each tablet contains 358 mg of creatine (calculated as creatine).
Embodiment 2
[0039] Example 2 Powder
[0040] Preparation of coating solution: weigh 3.3 kg of emulsion with 20% ethyl cellulose content, add 1.1 kg of water, stir for 0.5 hour, and set aside.
[0041] Weigh 5 kg of creatine ethyl ester and 6 kg of creatine monohydrate, and apply a top spray process in a fluidized bed for coating. (Parameters: Inlet air temperature 40°C, induced air volume 7m 3 / min, atomization pressure 0.2MPa, flow rate 10ml / min, operating time 65min, weight gain 5%)
[0042] The obtained sustained-release creatine powder has a percentage of creatine ethyl ester of 43.2%, a percentage of creatine monohydrate of 51.8%, and each gram of the product contains 0.80 g of creatine (calculated as creatine).
Embodiment 3
[0043] Example 3 Capsules
[0044] Preparation of coating solution: weigh 3.3 kg of emulsion with 20% ethyl cellulose content, add 1.1 kg of water, stir for 0.5 hour, and set aside.
[0045] Weigh 11 kg of creatine monohydrate, and apply a top spray process in a fluidized bed for coating. (Parameters: Inlet air temperature 40°C, induced air volume 3m 3 / min, atomization pressure 0.2MPa, flow rate 3ml / min, operating time 65min, weight gain 5%)
[0046] The above-mentioned granules are packed into capsule shells, and the amount of each granule is 600 mg.
[0047] The obtained sustained-release creatine granules have a percentage of creatine monohydrate of 95%, and each capsule contains 0.50 g of creatine (calculated as creatine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com