Nickle base catalyst for producing butane-1 by hydro-isomerization of butane-2
A nickel-based catalyst and hydroisomerization technology, which is applied in the fields of isomerization to hydrocarbon production, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc. Low conversion rate and other problems, to achieve the effect of low olefin hydrogenation rate, high equilibrium conversion rate and good pore size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
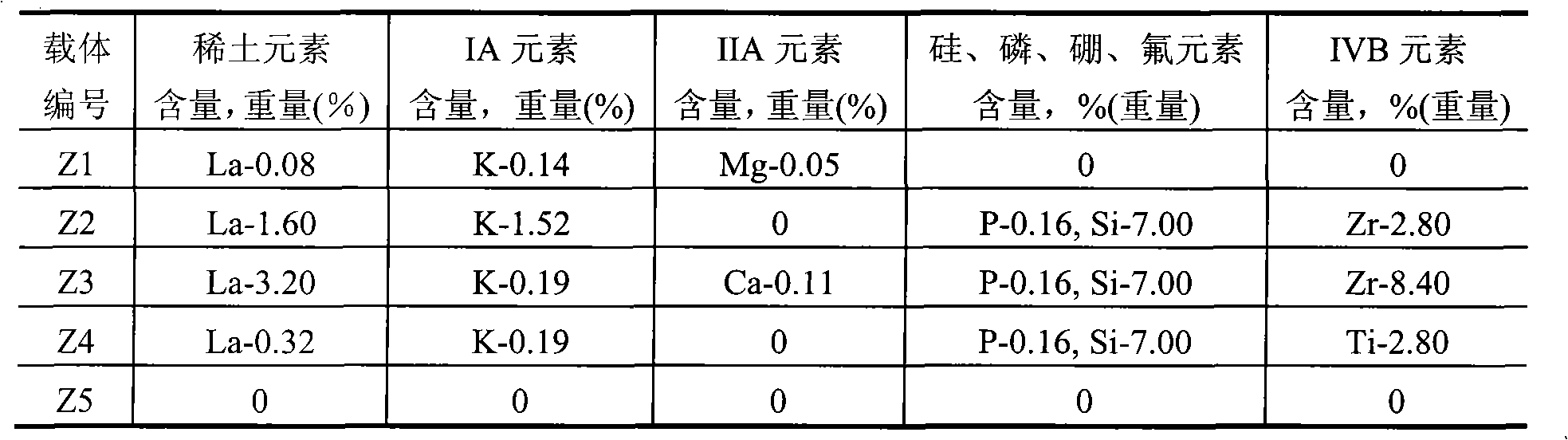
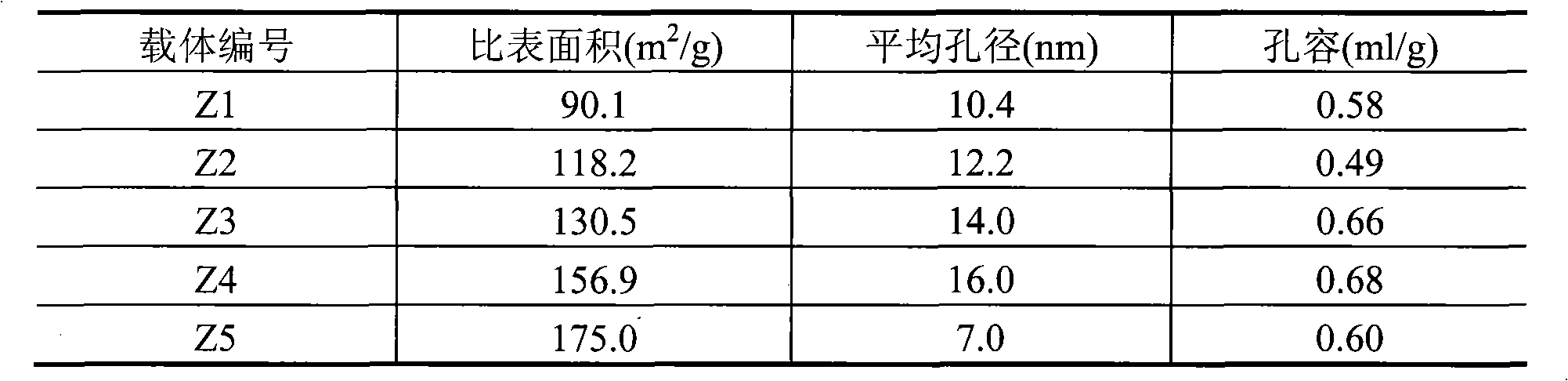
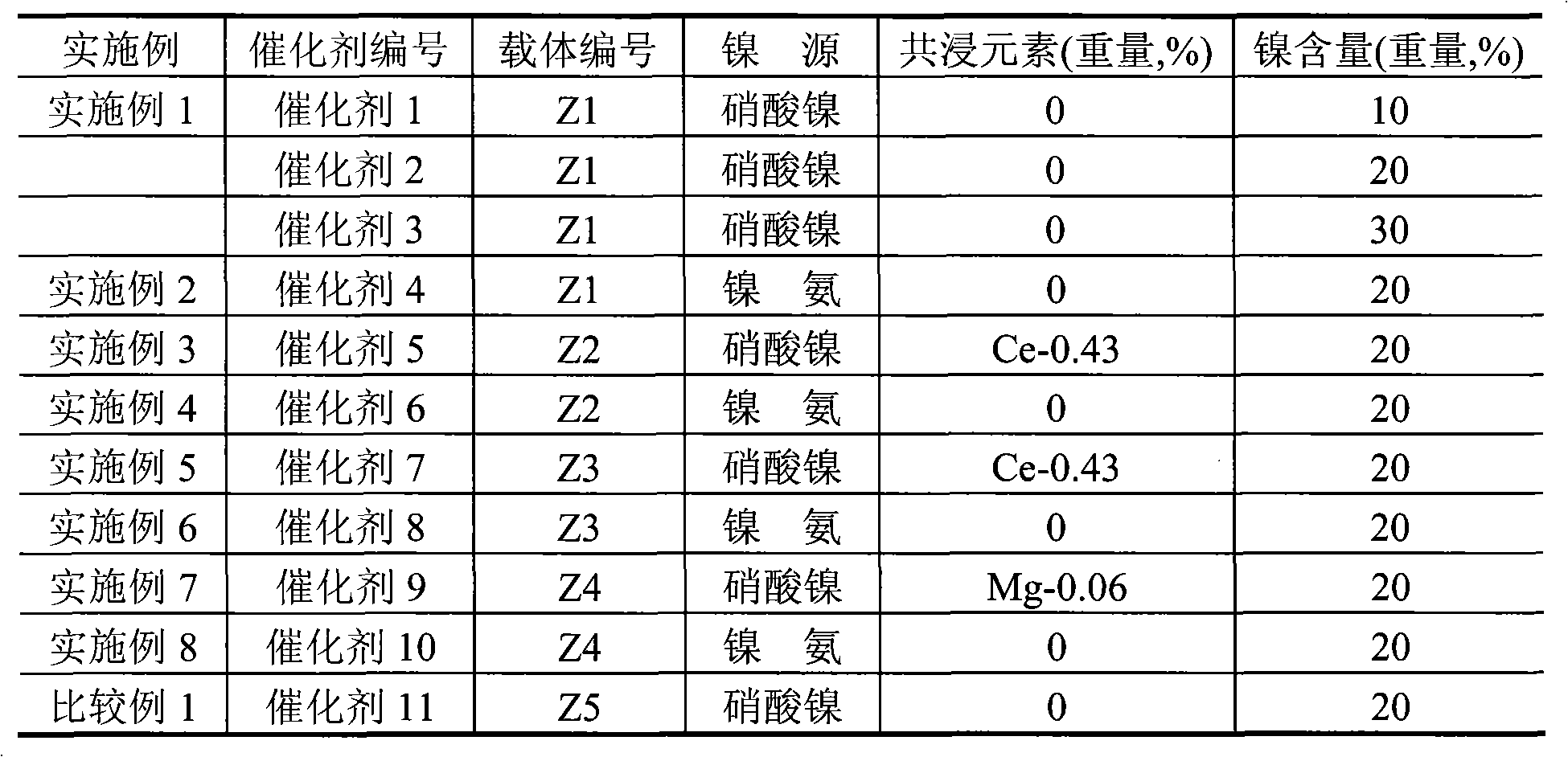
[0014] Take by weighing 300 grams of pseudo-boehmite, 150 grams of δ-alumina, 9 grams of scallop powder, mix, then add 25 grams of polyvinyl alcohol-containing solution (mass concentration is 5%), and concentration is 4.0 grams of 68% nitric acid , 1.5 grams of potassium nitrate, 2 grams of magnesium nitrate, 1.0 grams of lanthanum nitrate, 360 ml of aqueous solution, extruded into a clover-shaped carrier with a diameter of 2.5 mm, dried at 120°C for 4 hours and then roasted at 750°C for 4 hours to obtain carrier Z1 , the composition of the carrier is shown in Table 1, and the surface properties of the carrier are shown in Table 2. The carrier was impregnated in an equal amount of nickel nitrate impregnation solution with a metal nickel content of 14%, dried at 60°C for 8 hours, and calcined at 450°C for 4 hours to prepare Ni-based catalyst 1, so that the final Ni content was 10.0% of the weight of the carrier. The catalyst composition is shown in Table 3, and the content of e...
Embodiment 2
[0017] Carrier Z1 was used, and the preparation method of the carrier was the same as in Example 1, and the composition of the carrier was shown in Table 1. With the same operating steps and conditions of Example 1, just changing the nickel solution to be a nickel-ammonia complex solution (nickel carbonate: ammonium carbonate: ammoniacal liquor=1.0: 1.0: 1.0) with a metal nickel content of 12.0%, made Ni-based catalyst 4 , so that the final Ni content is 20.0% of the carrier weight. The catalyst composition is shown in Table 3, and the content of each component is based on the weight of the carrier.
Embodiment 3
[0019] Take by weighing 300 grams of pseudo-boehmite, 45 grams of diatomaceous earth, and 9 grams of asparagus powder, mix, then add 25 grams of polyvinyl alcohol solution (mass concentration is 5%), and the concentration is 4.0 grams of 68% nitric acid, 1.8 grams of phosphoric acid with a concentration of 85%, 12.0 grams of potassium nitrate, 40.0 grams of zirconium nitrate, 20.0 grams of lanthanum nitrate, 360 milliliters of aqueous solution, extruded into a clover carrier with a diameter of 2.5 mm, wet strips dried at 50 ° C for 24 hours and then dried at 750 ° C Calcined for 4 hours, the carrier Z2 was obtained. The composition of the carrier is shown in Table 1, and the surface properties of the carrier are shown in Table 2. Mix 3.0 grams of cerium nitrate with 50 grams of nickel nitrate solution with a metal nickel content of 14% to prepare an impregnation solution. The carrier and the impregnating solution were impregnated in equal amounts to prepare Ni-based catalyst 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com