Method for synthesizing glucurolactone
A synthetic method, glucuronolactone technology, applied in chemical instruments and methods, sugar compounds with non-glycosyl groups, drug combinations, etc., can solve the problems of many by-products, environmental pollution, waste of resources, etc., and achieve easy separation and purification , reduce the three wastes pollution, reduce the effect of nitrogen oxides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
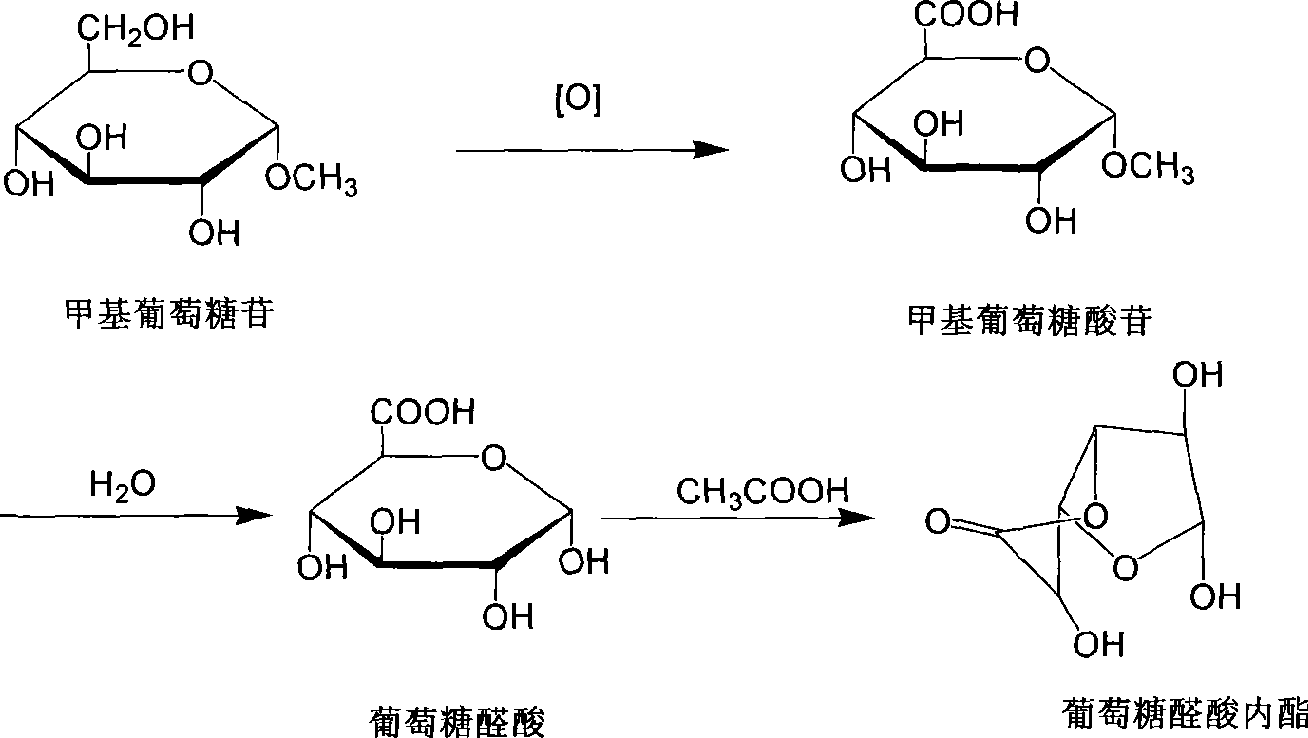
[0035] In 100ml of 0.5M sodium hydroxide, add 10g of methyl glucoside and stir to dissolve at 15°C; then add 1.5g of sodium bromide and 0.2g of catalyst TEMPO, stir to dissolve, cool to 5-10°C and add 120g of 12.5% sodium hypochlorite solution dropwise After the dropwise addition, keep the temperature for reaction for 6 hours, add 5 g of sodium thiosulfate to terminate the oxidation reaction, cool and filter to obtain the oxidation solution. In the oxidation solution, adjust the pH value to 1-2 with dilute hydrochloric acid. Heated to 85°C; hydrolyzed for 5 hours, and the liquid product was treated with a strong acidic cation exchange resin to remove metal ions to obtain a glucuronic acid solution; then concentrated to 30% of the volume, added 6 g of glacial acetic acid, heated to 50°C, and reacted for 8 hours; The reaction mixture was concentrated (40Be), cooled to 5°C, crystallized, filtered and dried to obtain 3.8 g of glucuronolactone. The yield was 41.9%; the content w...
Embodiment 2
[0037] In 100ml of 1M sodium bicarbonate, add 10g of methyl glucoside at 15°C and stir to dissolve; then add 1.5g of sodium bromide and 0.23g of catalyst 4-OH-TEMPO and stir to dissolve, cool to 15-20°C and add 120g of 12. 5% sodium hypochlorite solution, after the dropwise addition, keep the reaction at 30-40°C for 1 hour, add 5 g of sodium thiosulfate under cooling to terminate the oxidation reaction, cool and filter to obtain the oxidation solution. In the oxidation solution, adjust the pH value to 1-2 with dilute nitric acid. Heated to 90°C; hydrolyzed for 5 hours, and the liquid product was treated with a strong acidic cation exchange resin to remove metal ions to obtain a glucuronic acid solution; then concentrated to 30% of the volume, added 6 g of glacial acetic acid, heated to 55°C, and reacted for 6 hours; The reaction mixture was concentrated (39Be), cooled to 5°C, crystallized, filtered and dried to obtain 3.65 g of glucuronolactone. The yield was 40.2%; the conte...
Embodiment 3
[0039] In 100ml of 0.5M sodium bicarbonate, add 10g of methyl glucoside and stir to dissolve at room temperature at 16°C; add 50ml of dichloromethane, then add 1.5g of sodium bromide and 0.2g of catalyst TEMPO, stir to dissolve, drop at 30-40°C Add 100g30%H 2 o 2 After the solution was added dropwise, the reaction was kept for 8 hours, and 15 g of sodium thiosulfate was added to terminate the oxidation reaction, cooled and filtered; the organic layer was separated to obtain an oxidized solution. In the oxidation solution, adjust the pH value to 1-2 with dilute hydrochloric acid. Heat to 90°C; hydrolyze for 4 hours, and the liquid product is treated with a strong acidic cation exchange resin to remove metal ions to obtain a glucuronic acid solution; then concentrate to 30% of the volume, add 6 g of glacial acetic acid, heat up to 40°C, and react for 16 hours; The reaction mixture was then concentrated with 41Be, cooled to 0°C, crystallized, filtered and dried to obtain 3.9 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com