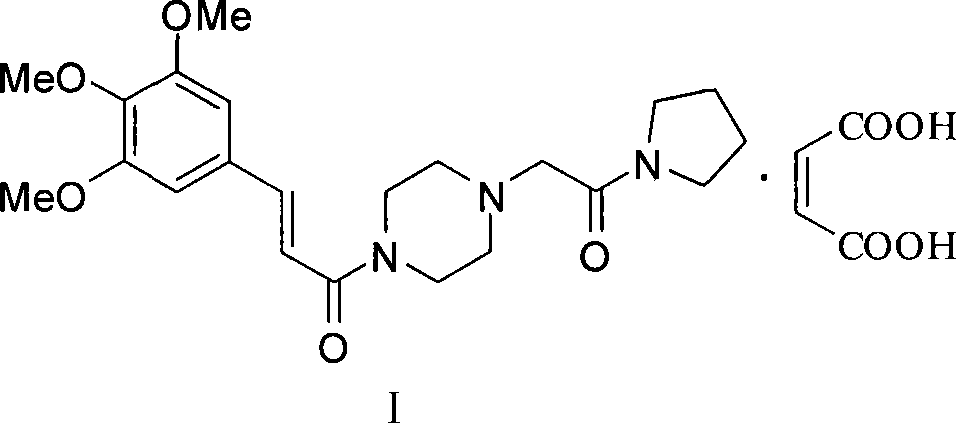
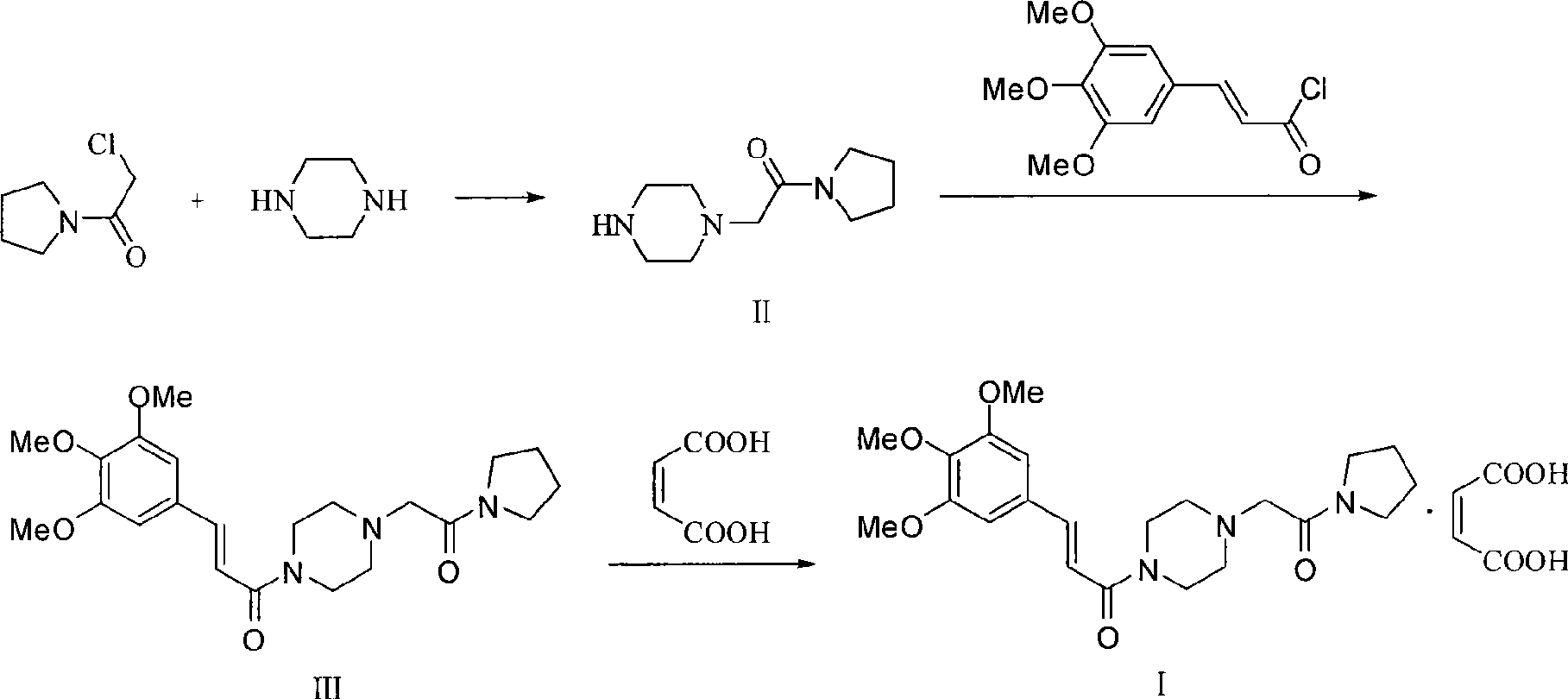
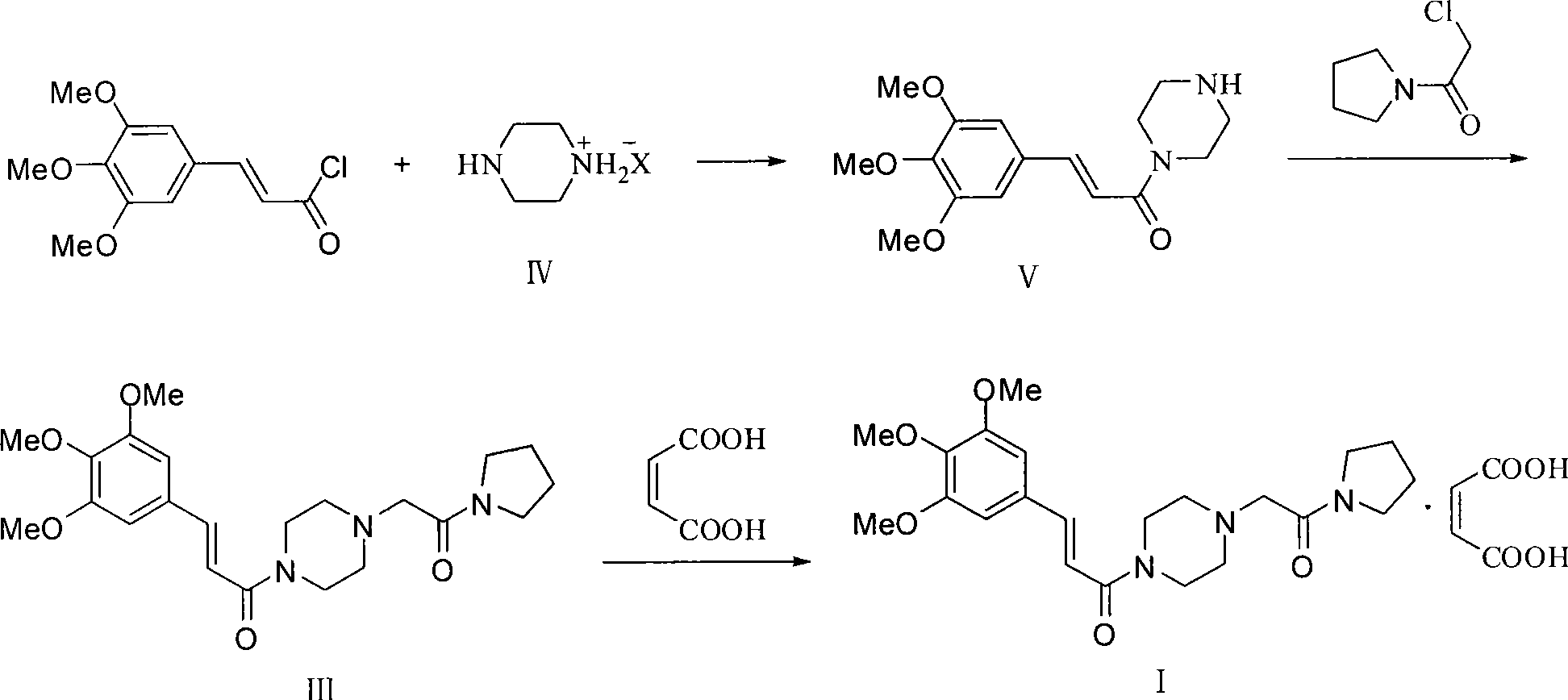
Improved preparation method for cinepazide maleate
A technology of cinepazide maleate and piperazine, which is applied in the field of preparation of cinepazide maleate to achieve the effects of mild reaction conditions, less by-products and reduced energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1) Preparation of 1-[(1-tetrahydropyrrolecarbonyl)methyl]piperazine
[0057] Reaction formula:
[0058]
[0059] At room temperature, add 11.7g (0.136mol) of anhydrous piperazine and 6.3g (0.045mol) of anhydrous potassium carbonate into 100ml of absolute ethanol and stir evenly. Then slowly add a solution of 6.6g (0.045mol) chloroacetylpyrrolidine and 30ml of absolute ethanol dropwise; keep warm for 3 to 4 hours (GC or TLC to follow the reaction process: methanol is the developer, iodine, chloroacetylpyrrolidine R f = 0.7, 1-piperazine acetylpyrrolidine R f =0.4), remove the solid residue by suction filtration, use 20ml acetone to stir at room temperature after 10min ice-water bath cooling after the obtained filtrate spins to dryness, remove the white powder (being piperazine, recycling) by suction filtration, after the filtrate spins to dryness Vacuum drying gave 8.4 g of a yellowish white solid with a yield of 95.6% and a GC purity of ≥99%.
[0060] 1 H-NMR (DM...
Embodiment 2
[0077] 1) Preparation of 1-[(1-tetrahydropyrrolecarbonyl)methyl]piperazine
[0078] Reaction formula:
[0079]
[0080] At room temperature, add 15.6g (0.181mol) of anhydrous piperazine into 100ml of absolute ethanol and stir evenly. Then slowly add a solution of 6.6g (0.045mol) chloroacetylpyrrolidine and 30ml of absolute ethanol dropwise; keep warm for 3 to 4 hours (GC or TLC to follow the reaction process: methanol is the developer, iodine, chloroacetylpyrrolidine R f = 0.7, 1-piperazine acetylpyrrolidine R f=0.4); after the reaction solution was evaporating to dryness, stir it with 20ml acetone at room temperature for 10min and then cool it in an ice-water bath, remove the white powder (being piperazine, recycle) by suction filtration, and vacuum-dry the filtrate to obtain a yellowish white color after evaporating to dryness. The solid is 8.8g, the yield is 100%, and the GC purity is 90.3%.
[0081] 1 H-NMR (DMSO-d 6 ): δ 1.747 (m, 2H, pyrrolidine-CH 2 -), δ 1.829...
Embodiment 3
[0098] 1) Preparation of 1-[(1-tetrahydropyrrolecarbonyl)methyl]piperazine
[0099] Reaction formula:
[0100]
[0101] At room temperature, 11.7g (0.136mol) of anhydrous piperazine and 6.3g (0.045mol) of anhydrous potassium carbonate were added to 100ml of absolute ethanol and stirred evenly. Then slowly add a solution of 6.6g (0.045mol) chloroacetylpyrrolidine and 30ml of absolute ethanol dropwise; keep warm for 3 to 4 hours (GC or TLC to follow the reaction process: methanol is the developer, iodine, chloroacetylpyrrolidine R f = 0.7, 1-piperazine acetylpyrrolidine R f =0.4), remove the solid residue by suction filtration, use 20ml acetone to stir at room temperature after 10min ice-water bath cooling after the obtained filtrate spins to dryness, remove the white powder (being piperazine, recycling) by suction filtration, after the filtrate spins to dryness Vacuum drying gave 8.4 g of a yellowish white solid with a yield of 95.6% and a GC purity of ≤99%.
[0102] 1 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com