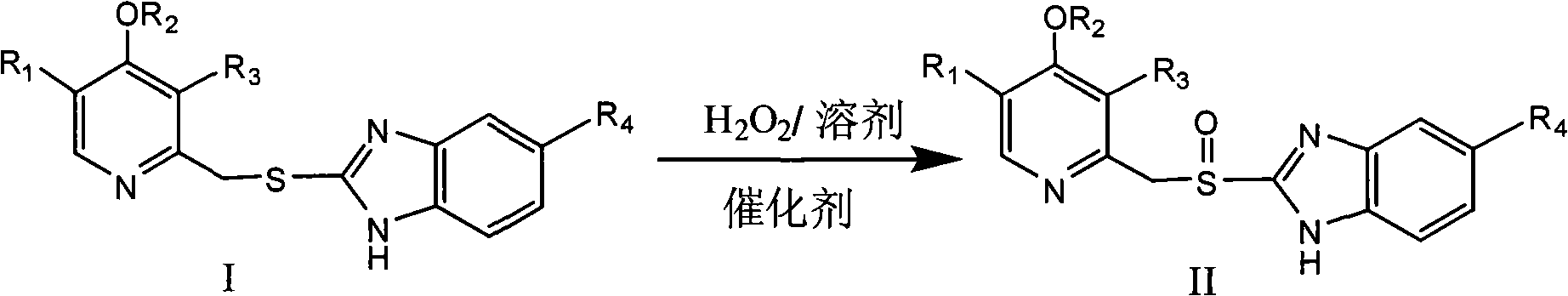
Novel method for preparing benzimidazole proton pump inhibitors
A technology of benzimidazole and solvent, which is applied in the field of preparation of benzimidazole proton pump inhibitors, can solve the problems of cumbersome post-processing, toxic and side effects of human body, complicated operation, etc., achieve good reaction efficiency and selectivity, and high production safety , high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
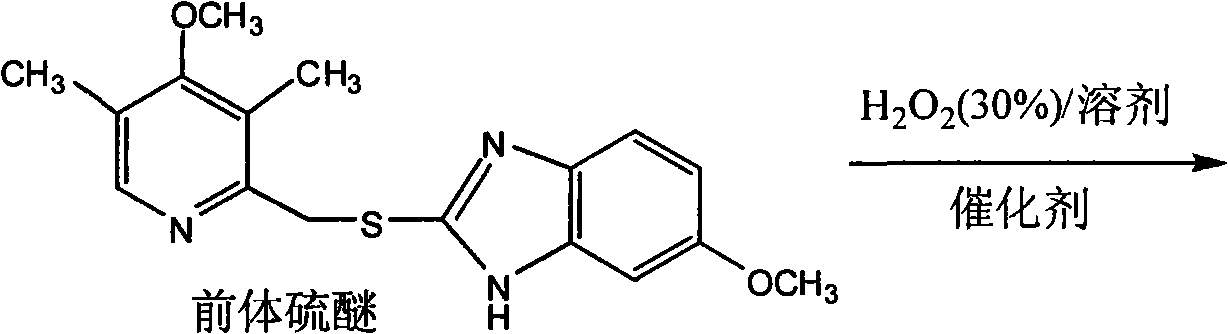
[0027] Embodiment 1 general operation:
[0028]
[0029]
[0030] When the temperature is 0°C, the catalyst (0.01 mmol, the number of moles is calculated according to the number of molybdenum atoms in the catalyst as 1) is added to 4 ml of solvent containing omeprazole precursor sulfide (0.5 mmol) (if the reaction To add the additive, it should be added in the above-mentioned organic solution in a certain molar ratio at this moment), after keeping the temperature constant and stirring for 30 minutes, slowly add 30% hydrogen peroxide solution (0.525 mmol) dropwise in the reaction solution, Stirring was continued at this temperature until the reaction was complete. Post-treatment to obtain a reaction mixture, wherein the components include omeprazole, its precursor thioether, peroxide sulfone and the like.
[0031] The determination of product content adopts the method of proton nuclear magnetic resonance spectroscopy, wherein the content is determined by using the extern...
Embodiment 2
[0032] The impact of the amount of embodiment 2 oxygenant 30% hydrogen peroxide on reaction:
[0033] The experimental operation is as described in Example 1, and the molar ratio of feeding is as follows: the molar number of omeprazole precursor sulfide is 1, and the molar number of ammonium heptamolybdate is 0.02 (this molar number is calculated with the molar number of molybdenum atoms in the molecular formula ), the number of moles of hydrogen peroxide is X. The reaction uses ethanol as the reaction solvent and 30% hydrogen peroxide as the oxidant. When the amount X of hydrogen peroxide was changed, the experimental results obtained are shown in Table 1.
[0034] Table 1
[0035]
[0036] Note: 1) The data of conversion, yield, and sulfoxide / sulfone ratio were obtained from 1 H NMR determination, wherein the conversion rate was obtained by adding quantitative dimethyl maleate (DMM) as an external standard compound.
[0037] 2) The sulfoxide is omeprazole, and the sul...
Embodiment 3
[0039] The influence of embodiment 3 different catalysts on oxidation reaction:
[0040] The experimental operation is as described in Example 1, and the molar ratio of feeding is as follows: Omeprazole precursor sulfide / catalyst / hydrogen peroxide=50 / 1 / 52.5 (when polyoxometalate is used as catalyst, its mol ratio is based on metal atom calculated in terms of moles). The reaction takes ethanol as the reaction solvent, 30% hydrogen peroxide solution as the oxidant, and the time is 36 hours. By examining the data obtained from the catalytic reactions of different catalysts, the catalysts with excellent performance were screened out. The obtained experimental results are shown in Table 2.
[0041] Table 2
[0042]
[0043] Note: 1) The data of conversion, yield, and sulfoxide / sulfone ratio were obtained from 1 H NMR determination, wherein the conversion rate was obtained by adding quantitative dimethyl maleate (DMM) as an external standard compound.
[0044] 2) No. 1 is th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com