Fluorescent cell model for screening M2 ion channel blocker for influenza virus and application method thereof
A cell model and ion channel technology, applied in the field of genetic engineering, can solve problems such as difficult high-throughput screening, limited ion channel research methods, and many influencing factors, achieving high-throughput accurate screening, clear drug action mechanism, and simple methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
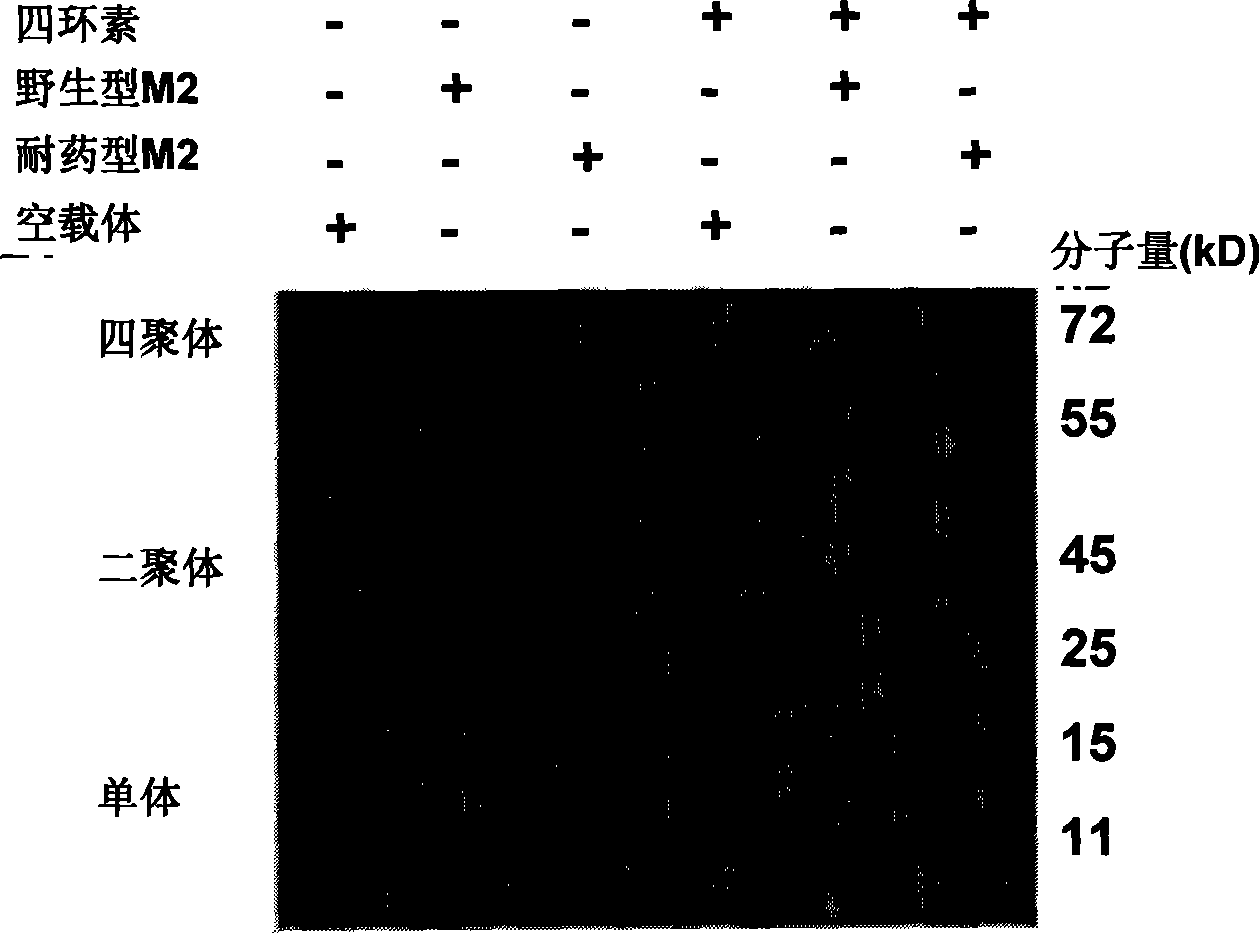
[0030] Example 1: Establishment of co-expression cells of H5N1 influenza A virus M2 ion channel and enhanced green fluorescent protein (EGFP)
[0031] 1. Acquisition of M2 ion channel protein gene of H5N1 influenza A virus
[0032] Using the consensus amino acid sequence of the H5N1 virus strain as a template, after optimization, the gene sequence suitable for mammalian expression was artificially synthesized (Guangzhou Funeng Gene Co., Ltd.), and named H5M2. At the same time, an expression plasmid of a drug-resistant mutant (S31N-L26I double mutation) of H5N1 influenza virus M2 was constructed, and the plasmid was named H5M2 (S31N-L26I). Both were digested with BamHI and XbaI and cloned into pcDNA4 / TO plasmid, which can only express the target gene under the induction of tetracycline in T-REx293 cells. The wild-type H5M2 gene sequence is:
[0033] CGG GAT CCAT gtc cct gct gac aga ggt gga gac ccc cac cag gaa tga gtg gga gtg cag
[0034] gtg ctc tga ctc ctc tga CCC CCT GGT G...
Embodiment 2
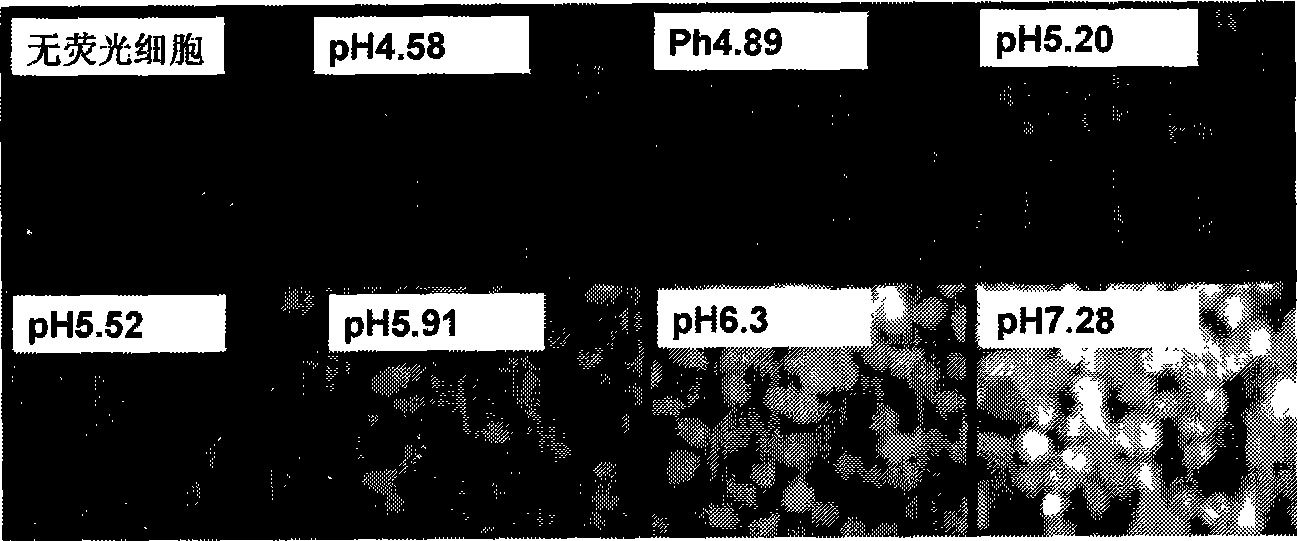
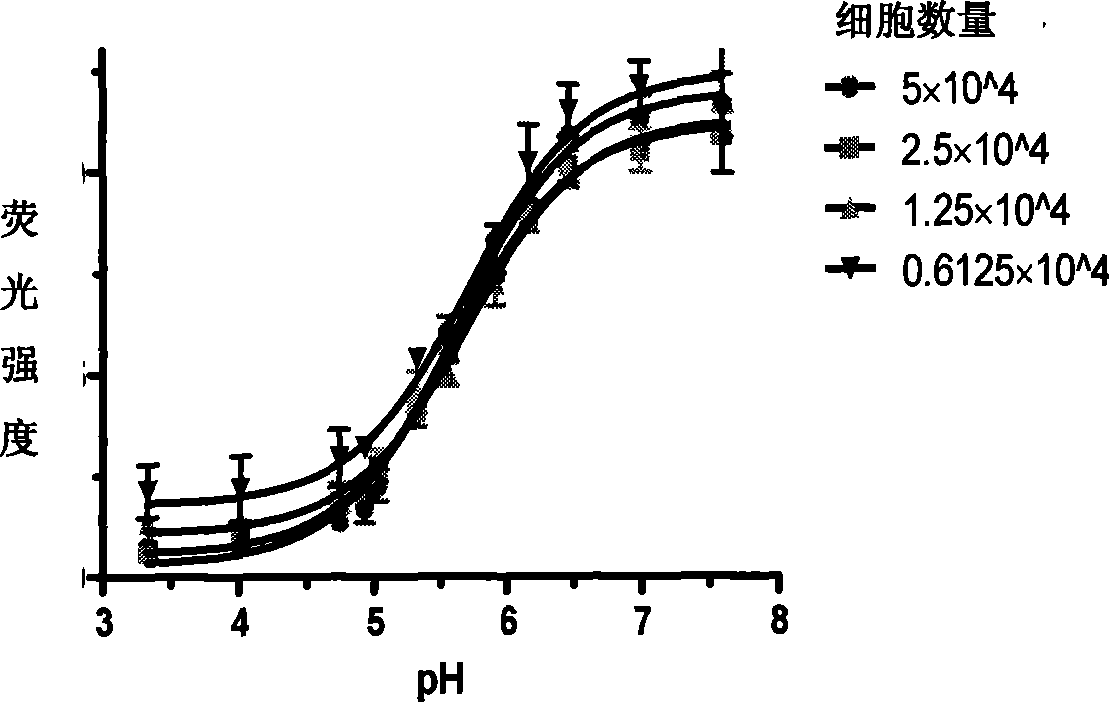
[0140] Example 2. Fluorescent indicator labeled M2 expressing cell model
[0141] 1. Take 2×10 cells of M2T-REx293 stable strain in good condition 6 One was inserted into a 10cm culture dish (wild type and mutant type), induced by tetracycline 2 μg / ml and cultured for 24h; in addition, the cells of the same stable strain were put into two 10cm culture dishes (wild type and mutant type), without induction, as a control . Add 10ml of complete medium and place in a carbon dioxide incubator at 37°C and 5% CO2 for constant temperature cultivation.
[0142] 2. After 24 hours, the cells were collected without trypsinization, and the supernatant was removed. The cells were gently blown down with 1 ml of PBS (0.01M, pH 7.4) buffer, and collected into a 1.5 ml EP tube. Cells were washed three times with PBS pH 7.4.
[0143] 3. The cells were resuspended with 1 ml of Hanks buffer, and labeled with biscarboxyethylcarboxyfluorescein tetraacetoxymethyl ester (BCECF-AM, final concentratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com