Influenza virus H1N1 subtype neuraminidase as well as gene, inhibitor screening model and application thereof
A neuraminidase, H1N1 technology, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve the problems of tedious and time-consuming preparation of neuraminidase, safety issues, etc. Sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
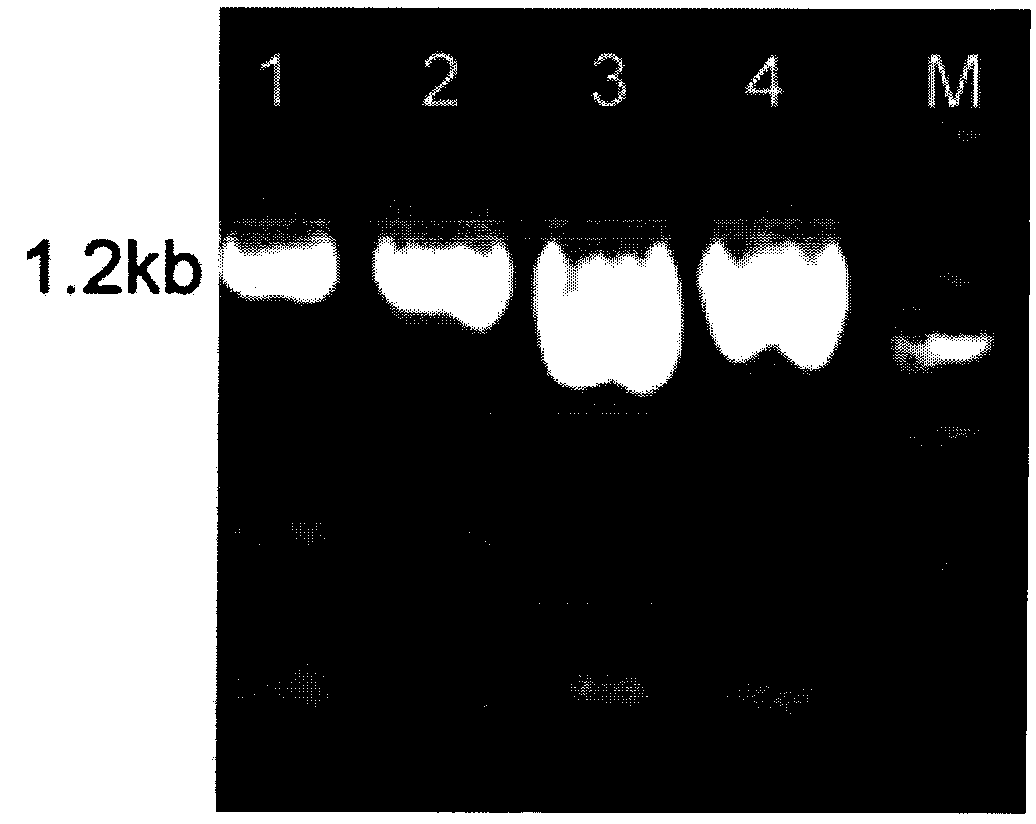
[0031] Example 1 Construction of recombinant plasmid pPICZαA-NAS
[0032] 1) Obtained 1629 neuraminidase amino acid sequences of influenza A virus H1N1 subtypes reported from April 2009 to March 2010 from the NCBI website, and selected the most representative virus strains after comparison and analysis by CluxtalX software A / Wisconsin / 629-S0247 / 2009 (H1N1) (GenBank: CY051377) was used as the research object. The full-length nucleic acid of the gene is 1407bp, encoding 469 amino acids. According to the expression habit of Pichia pastoris, the gene is codon-optimized, and the sequence of the codon-optimized NA full-length gene is shown in SEQ ID NO.1 in the sequence table. The optimized gene was synthesized by chemical synthesis, and the synthesized full-length gene was connected to the cloning vector pUC57 to obtain the cloning vector pUC57-NA, and the sequence was verified to be completely correct (entrusted to Shanghai Jingjing Biosynthesis).
[0033] 2) Design primers for P...
Embodiment 2
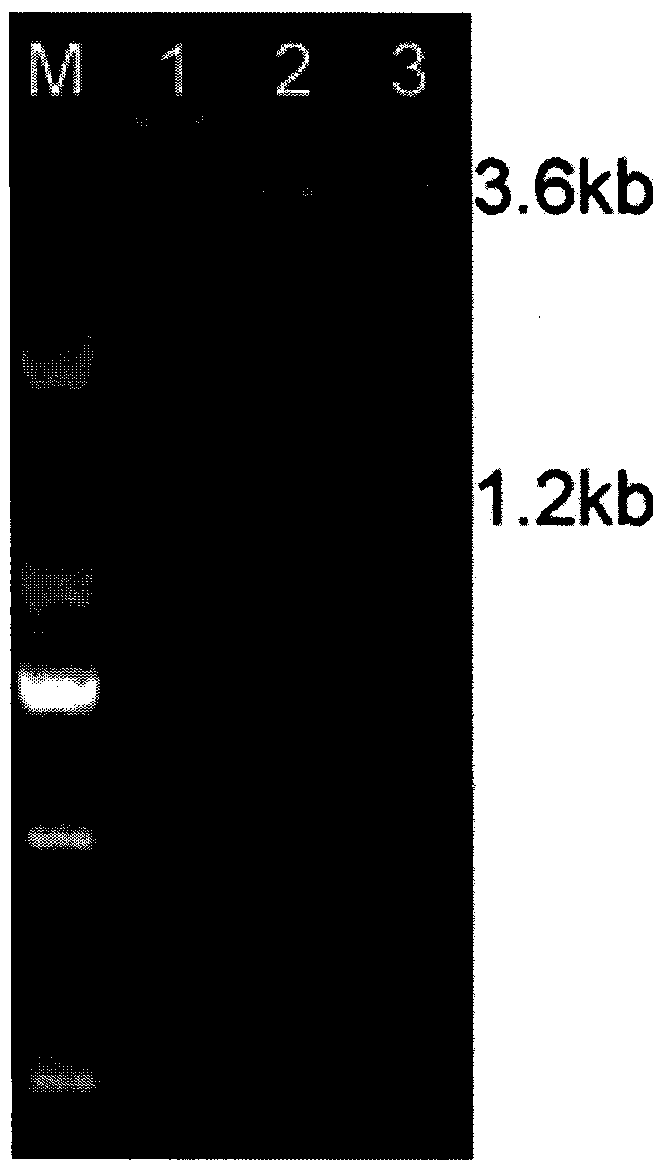
[0037] Example 2 Construction of recombinant Pichia pastoris strain and induced expression of truncated NA
[0038] 1) Prepare KM71 and SMD1168 competent, the specific steps include:
[0039] a. Single colonies of KM71 (purchased from Invitrogen) and SMD1168 (purchased from Invitrogen) grown on YPD plates at 30° C. were picked and inoculated into 20 mL of YPD liquid medium at 250 rpm at 30° C. for overnight cultivation.
[0040] b. Take 0.1-0.5mL overnight culture, inoculate a 2L shake flask containing 500mL fresh medium, and culture overnight until OD600=1.3-1.5.
[0041] c. Collect the cells by centrifugation at 1500g for 5 minutes at 4°C, and suspend the cells with 500 mL of pre-cooled sterilized water.
[0042] d. Centrifuge as above, and suspend the cells with 250 mL of pre-cooled sterile water.
[0043] e. Centrifuge as above, and suspend the cells with 20 mL of pre-cooled 1M sorbitol.
[0044] f. Centrifuge as above, and suspend the cells with 1 mL of pre-cooled 1M s...
Embodiment 3N
[0054] Embodiment 3NA activity measurement and inhibition experiment
[0055] 0.5% methanol induced recombinant Pichia pastoris to secrete and express truncated NA, and the bacterial solution induced for 96 hours was centrifuged at 12000rpm for 5min, and the supernatant was taken as the crude enzyme solution for NA activity assay, and at the same time, it was concentrated by ultrafiltration in a centrifuge tube. The crude enzyme solution was concentrated about 10 times and used for the inhibition experiment of oseltamivir, zanamivir, amantadine and rimantadine on truncated NA. The experimental equipment was BioTek multifunctional microplate reader and 96-well black microplate plate .
[0056] NA activity assay: take 30 μl fermentation supernatant, add 75 μl reaction substrate (final concentration is 32.5mM MES buffer, pH6.5, 4mM CaCl 2 , 100 μM substrate 4-MUNANA), the kinetic method detects the change of fluorescence intensity under the excitation light of 360nm and the emis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com