Method for oxidation synthesis of stilbenes by hexamethylenetetramine
A technology of hexamethylene tetramine and stilbene, which is applied in the field of synthesizing stilbene compounds by oxidation of hexamethylene tetramine, can solve the problems of difficulty in realizing industrialized production, high toxicity, environmental damage and the like, and achieves the The method is simple and easy to implement, the cost is low, and the yield is high.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
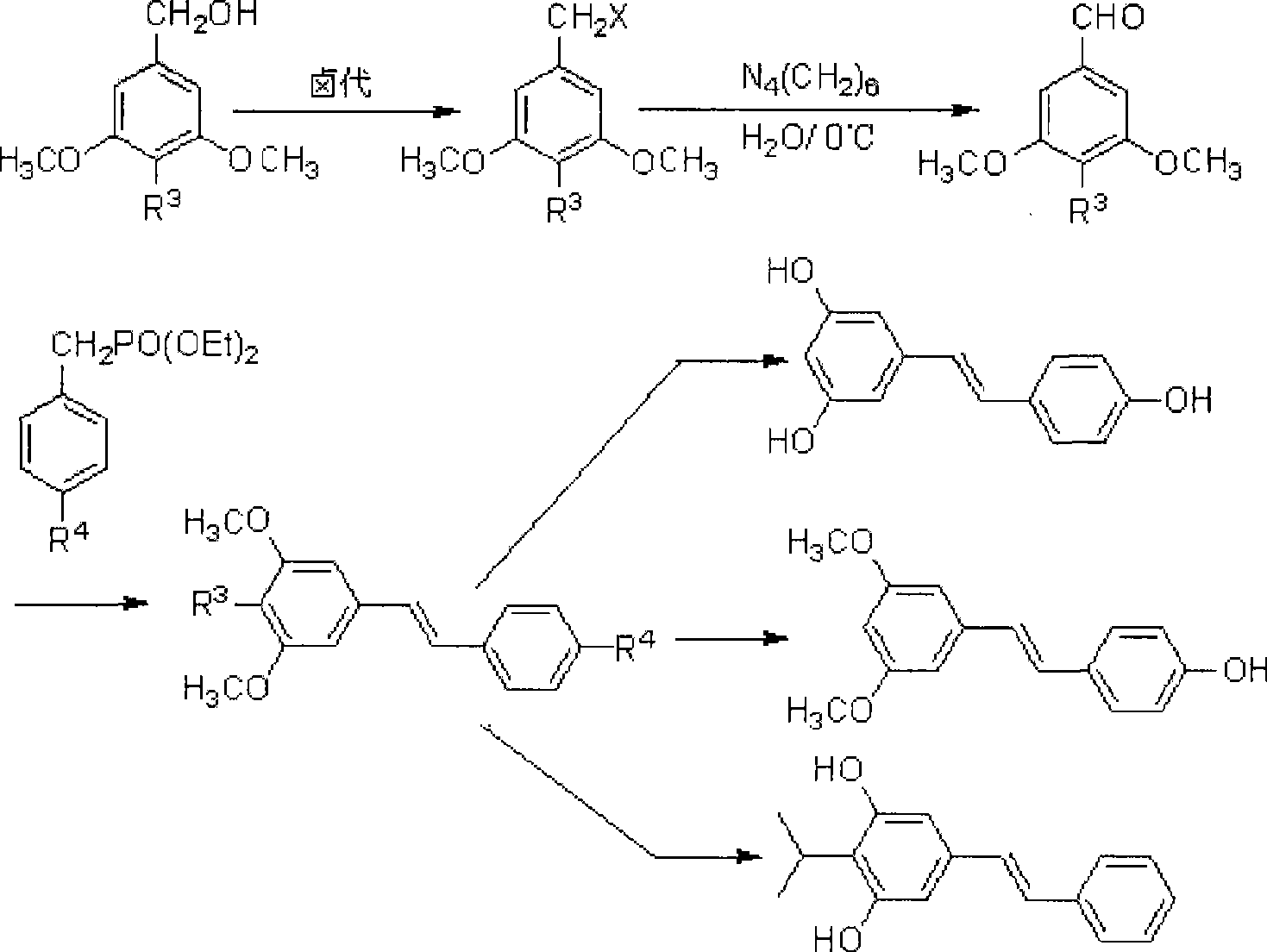
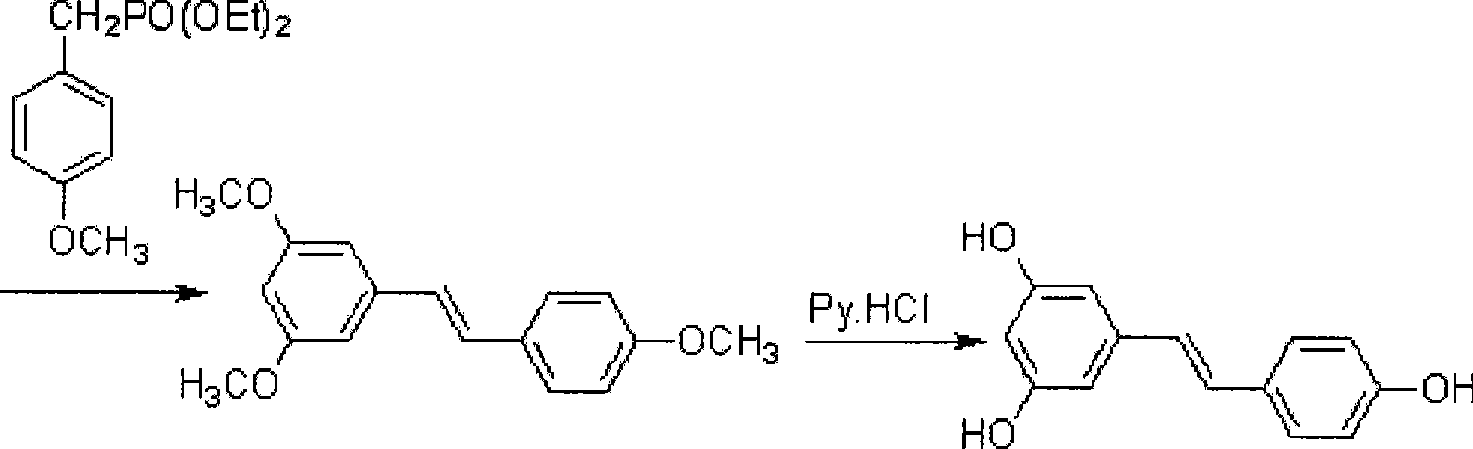
[0050] This example is a method for synthesizing 3,4′,5-trihydroxystilbene, namely resveratrol, by oxidation of hexamethylenetetramine, the reaction route of which is shown in formula (II):
[0051]
[0052] Formula (II)
[0053] The reaction process is a1→b1→c1→d1, and the reaction formula of each step is as follows:
[0054]
[0055]
[0056] This method is carried out according to the following sequence of steps:
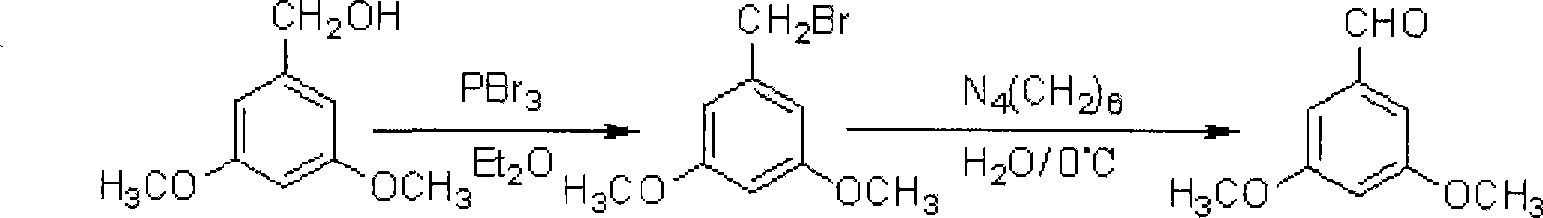
[0057] a 1 . Compound A1 3, the preparation of 5-dimethoxybenzyl bromide
[0058] Take 10.60g (63.10mmol) of 3,5-dimethoxybenzyl alcohol and 100mL of diethyl ether into a 250mL four-neck flask, stir, cool down, add 7.10mL (75.72mmol) of phosphorus tribromide dropwise at 0°C, and add dropwise After completion, it was raised to room temperature for reaction and monitored by TLC. After the reaction was completed, the reaction solution was poured into ice water, stirred, and the organic layer was separated, washed with water, dried, suction filtered, and...
Embodiment 2
[0066] This example is a method for the oxidation of hexamethylenetetramine to synthesize 3,5-dimethoxy-4'-hydroxystilbene, that is, pterostylbene, and its reaction route is as shown in formula (III):
[0067]
[0068] Formula (III)
[0069] The reaction process is a2→b2→c2→d2, and the reaction formula of each step is as follows:
[0070]
[0071]
[0072] This method is carried out according to the following sequence of steps:
[0073] a 2 .3, the preparation of 5-dimethoxybenzyl chloride namely A2:
[0074] Add 10.82g (64.40mmol) of 3,5-dimethoxybenzyl alcohol and 110mL of dichloromethane into a 250mL four-neck flask, stir, cool down, and add 5.61mL (77.28mmol) of thionyl chloride dropwise at 0°C, dropwise After the addition was completed, it was raised to room temperature for reaction, and monitored by TLC. After the reaction was completed, water was added, the organic layer was separated, washed with water, dried, filtered with suction, and the solvent was rem...
Embodiment 3
[0082] This example is a method for oxidizing hexamethylenetetramine to synthesize 3,5-dimethoxy-4-isopropyl stilbene, that is, styrene moder. The reaction route is as follows:
[0083]
[0084] Formula (IV)
[0085] The reaction process is a3→b3→c3→d3, and the reaction formula of each step is as follows:
[0086]
[0087] This method is carried out according to the following sequence of steps:
[0088] a 3 .Compound A3 is the preparation of 3,5-dimethoxy-4-isopropylbenzyl chloride:
[0089] Take 10.81g (51.48mmol) of 3,5-dimethoxy-4-isopropylbenzyl alcohol and 110mL of dichloromethane into a 250mL four-neck flask, stir, cool down in an ice bath, and add chlorinated chlorinated dropwise at 0°C. Sulfone 4.50mL (61.77mmol), rise to room temperature reaction after dropwise addition, TLC monitoring. After the reaction was completed, water was added, extracted with dichloromethane, washed with water, and the solvent was removed by rotary evaporation to obtain 10.00 g (43....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com