A medical equipment carrying extracellular matrix and its production method
A technology of medical devices and extracellular matrix, which is applied in the field of medical devices to achieve the effect of promoting endothelialization, reducing restenosis rate, and accelerating surface endothelialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
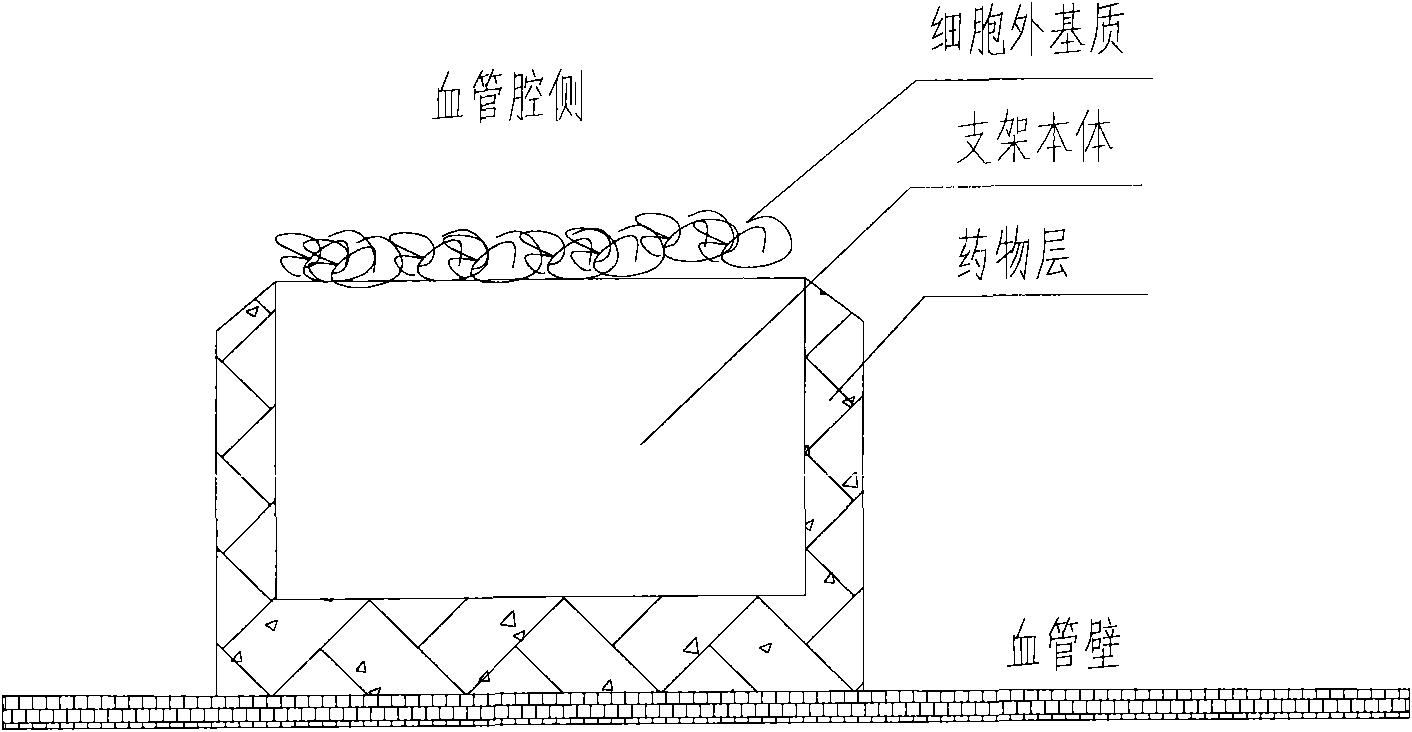
[0049] Preparation of scaffolds carrying extracellular matrix (collagen I and laminin 1), the cross-sectional view of which is shown in figure 2 Shown:
[0050] (1) The bare 316L stainless steel stent was cut, slag-removed, polished, placed in 75% medical alcohol, cleaned with ultrasonic waves at a frequency of 30khz for 15 minutes, set at 40°C, dried for 60 minutes, and then taken out.
[0051] (2) Put the scaffold into a 5mL glass test tube (1 support corresponds to 1 test tube), then add 1mL collagen I (concentration: 150μg / mL, solvent: 12M HCl) to the glass test tube, and add 1mL laminin at the same time 1 (the concentration is 10 μg / mL, the solvent is 0.1 MCBC buffer solution, pH 9.0), shake at 4°C for 1 hour, and take it out.
[0052] (3) The scaffolds coated with collagen I and laminin 1 were placed in an ultraviolet curing spotlight DymaxTM Bluewave 200 for 30 seconds, then rinsed and dried with deionized water, and dried at room temperature.
Embodiment 2
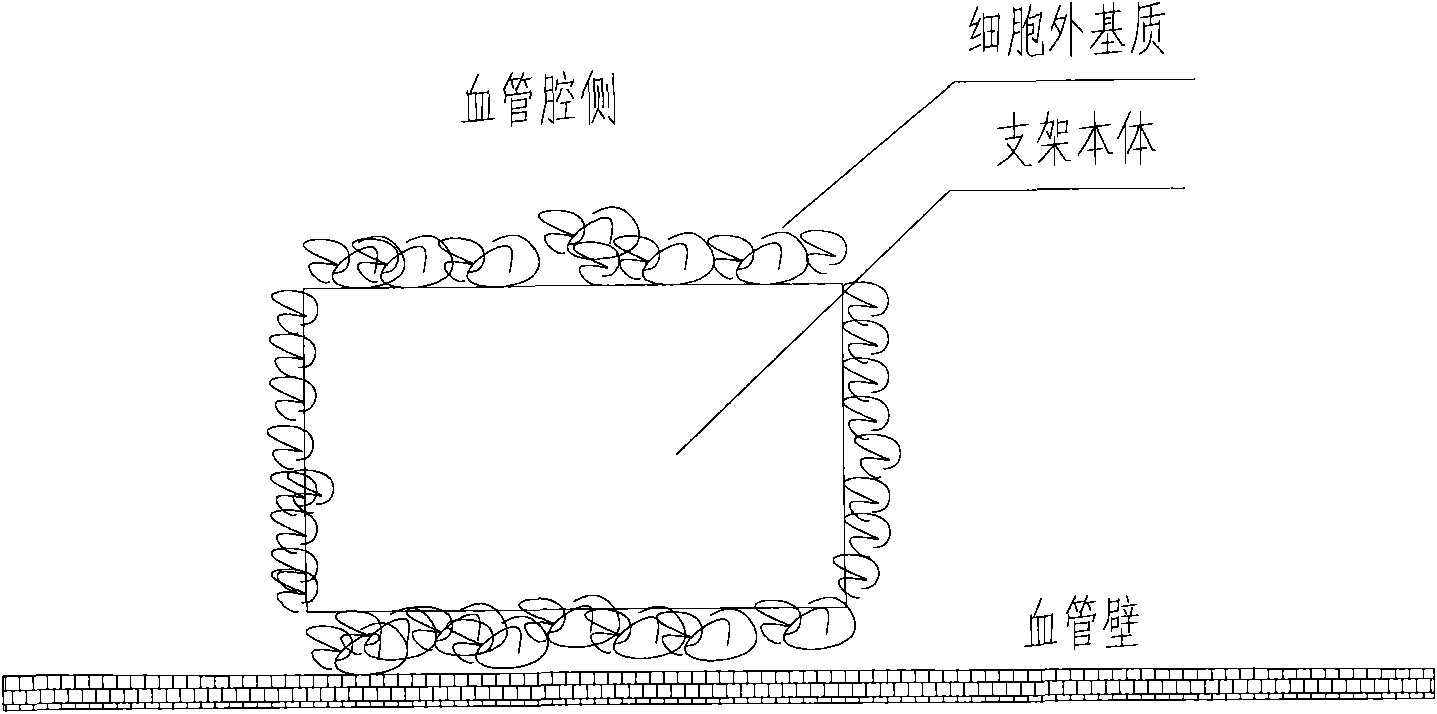
[0054] Preparation of extracellular matrix (collagen I and laminin 5) coated scaffolds, the cross-sectional view of which is shown in image 3 Shown:
[0055] (1) The bare 316L stainless steel stent was cut, slag-removed, polished, placed in 75% medical alcohol, cleaned with ultrasonic waves at a frequency of 30khz for 15 minutes, set at 40°C, dried for 60 minutes, and then taken out. Then place the bracket in ~38% hydrochloric acid for 12 hours of corrosion, connect the bracket body as the anode to the positive pole of the pulse power supply, connect the titanium metal sheet to the negative pole of the pulse power supply as the cathode, and place the bracket body and the cathode metal sheet at the same time. In 28% hydrochloric acid, the current is set to 20A, the frequency is 2000 Hz, and the time is 5 minutes, holes are prepared on the surface of the 316L bare metal stent body.
[0056] (2) Put the scaffold into a 5mL glass test tube (1 support corresponds to 1 test tube),...
Embodiment 3
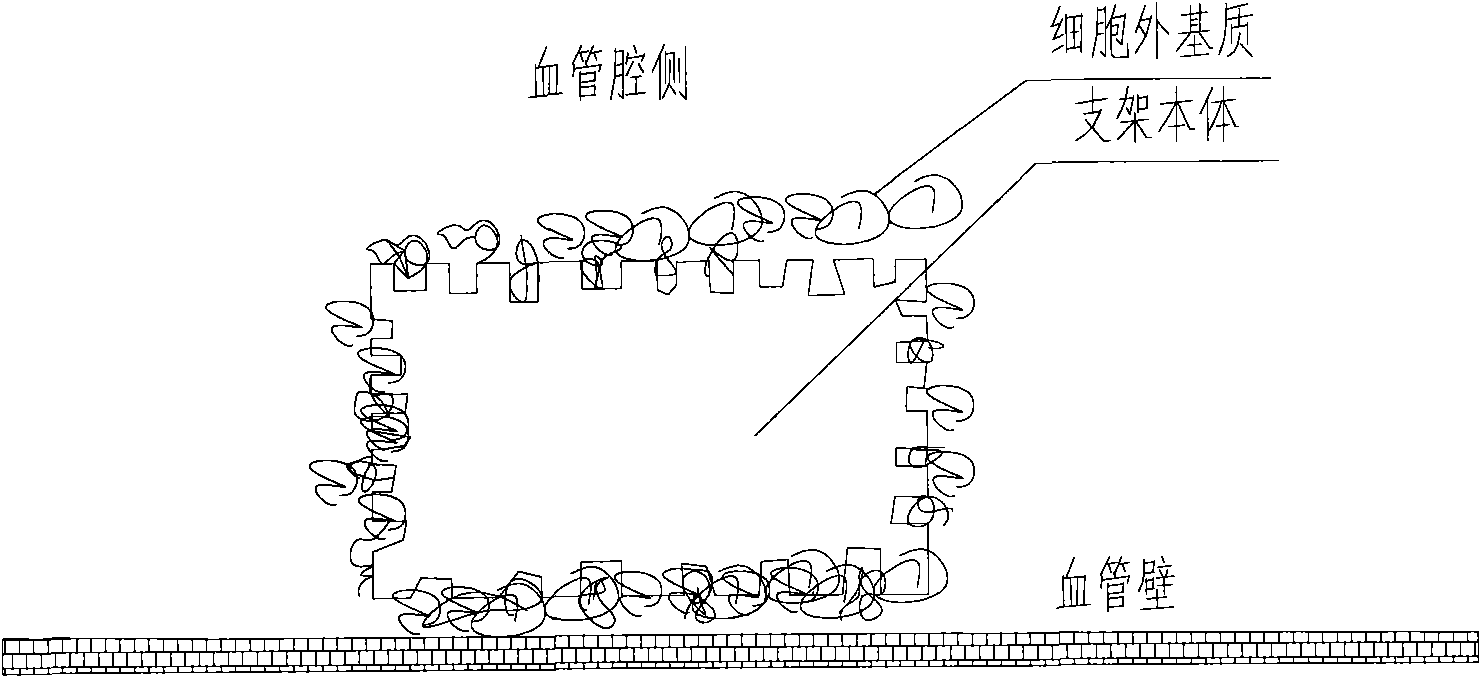
[0058] Prepare a kind of extracellular matrix (collagen I and laminin 1) drug-loaded (rapamycin) coated stent, its cross-sectional view is as follows Figure 4 Shown:
[0059] (1) The bare 316L stainless steel stent was cut, slag-removed, polished, placed in 75% medical alcohol, cleaned with ultrasonic waves at a frequency of 30khz for 15 minutes, set at 40°C, dried for 60 minutes, and then taken out.
[0060] (2) Add 0.8g polylactic acid into 10ml tetrahydrofuran solution, add 0.2g rapamycin after dissolving, then spray on the surface of the stent body, and solidify in the air for 60min, so that the rapamycin drug loading capacity of the stent reaches 1.2 μg / mm2.
[0061] (3) Then put the prepared drug stent into a 5mL glass test tube (one stent corresponds to one test tube), and then add 1mL collagen I (concentration: 150μg / mL, solvent: 12M HCl) into the glass test tube, and at the same time Add 1 mL of laminin 1 (concentration: 10 μg / mL, solvent: 0.1 M CBC buffer, pH 9.0)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com