L-alpha-amino acid ester contained Z-configuration anabasine pesticide and preparation method thereof
A neonicotinoid, amino acid ester technology, applied in the directions of insecticides, biocides, biocides, etc., can solve problems such as the production and use of pesticides that have never been seen in neonicotinoid insecticides, and achieve obvious novelty and efficiency. Creativity, remarkable utility, high yield and high purity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
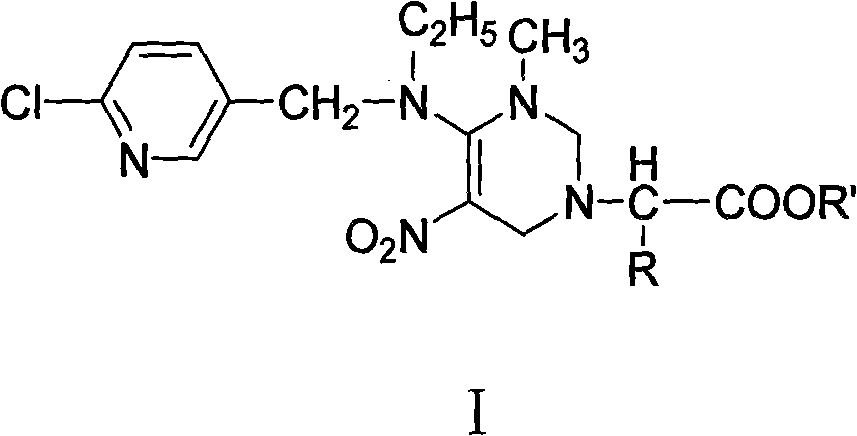
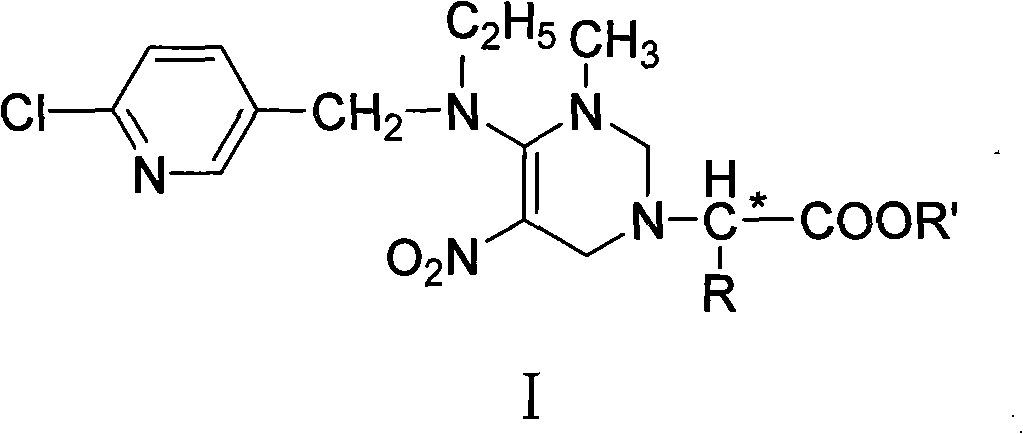
[0050] Preparation of (2H,6H)-4-[[(6-chloro-3-pyridylmethyl)ethylamino]-2,6-dihydro-3-methyl-5-nitro]-1-pyrimidineacetic acid ethyl Esters (Ia):
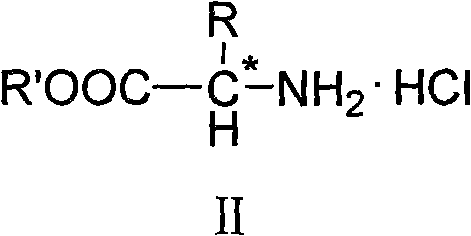
[0051] Put 20mL of absolute ethanol and 3.76g of glycine in a 100mL round-bottomed three-necked flask, cool it with an ice-salt bath, add 6mL of freshly distilled thionyl chloride dropwise, keep the temperature below -5°C, and add in about 30 minutes. Finish. After naturally rising to room temperature, it was heated to reflux until all the solids were dissolved, continued to reflux for 15 minutes, and then cooled slightly to obtain a white foamy solid. Remove excess thionyl chloride under reduced pressure, wash with diethyl ether and filter with suction after cooling, and recrystallize with absolute ethanol-diethyl ether to obtain white needle-like crystals (IIa).
[0052] Dissolve 5.30g of nitenpyram, 3.33g of ethyl glycine hydrochloride, 3.3mL of triethylamine and 3.8mL of 37% formaldehyde solution in 30mL of ethanol, and place ...
Embodiment 2
[0057] Preparation of (2H,6H)-4-[[(6-chloro-3-pyridylmethyl)ethylamino]-2,6-dihydro-3-methyl-5-nitro]-1-pyrimidine-( 1'-isopropyl)-ethyl acetate (Ib):
[0058] Put 20mL of absolute ethanol and 5.85g of L-α-leucine in a 100mL round-bottomed three-neck flask, cool with an ice-salt bath, add 6mL of freshly distilled thionyl chloride dropwise, and control the temperature at -5°C Below, it takes about 30 minutes to add. After naturally rising to room temperature, it was heated to reflux until all the solids were dissolved, continued to reflux for 15 minutes, and then cooled slightly to obtain a white foamy solid. Remove excess thionyl chloride under reduced pressure, wash with diethyl ether and filter with suction after cooling, and recrystallize with absolute ethanol-diethyl ether to obtain white needle-like crystals (IIb).
[0059] Dissolve 5.30g of nitenpyram, 4.67g of L-α-leucine ethyl ester hydrochloride, 3.3mL of triethylamine and 3.8mL of 37% formaldehyde solution in 30mL ...
Embodiment 3
[0065] Preparation of (2H,6H)-4-[[(6-chloro-3-pyridylmethyl)ethylamino]-2,6-dihydro-3-methyl-5-nitro]-1-pyrimidine-( 1'-benzyl)-ethyl acetate (Ic);
[0066] Put 20mL of absolute ethanol and 8.25g of L-α-phenylalanine in a 100mL round-bottomed three-neck flask, cool with an ice-salt bath and add 6mL of freshly distilled thionyl chloride dropwise, and control the temperature at -5 Below ℃, add in about 30 minutes. After naturally rising to room temperature, it was heated to reflux until all the solids were dissolved, continued to reflux for 15 minutes, and then cooled slightly to obtain a white foamy solid. Remove excess thionyl chloride under reduced pressure, wash with diethyl ether and filter with suction after cooling, and recrystallize with absolute ethanol-diethyl ether to obtain white needle-like crystals (IIc).
[0067] Dissolve 5.30g of nitenpyram, 5.47g of L-α-phenylalanine ethyl ester hydrochloride, 3.3mL of triethylamine and 3.8mL of 37% formaldehyde solution in 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com