Method for preparing nitrogen doped nanometer titanium dioxide visible light photocatalyst
A nano-titanium dioxide, photocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of high drying temperature, reduced catalytic activity, easy collapse of pores, etc., and increase the diameter of pores. , The effect of increasing the specific surface area and preventing collapse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Configure 0.01mol / L titanium sulfate solution, add surfactant triethanolamine under stirring condition, the quality of surfactant accounts for 0.1% of the quality of titanium sulfate solution, add 1mol / L ammonia water after stirring evenly, make sulfuric acid Titanium is completely precipitated, continue to stir for 0.5h, and let stand and age for 20h;
[0023] 2) washing the precipitate in step 2) with deionized water until the total concentration of anions in the washed water is lower than 0.1mol / L, and then vacuum-drying the precipitate in an environment with a pressure below 1000Pa and a temperature below -10°C;
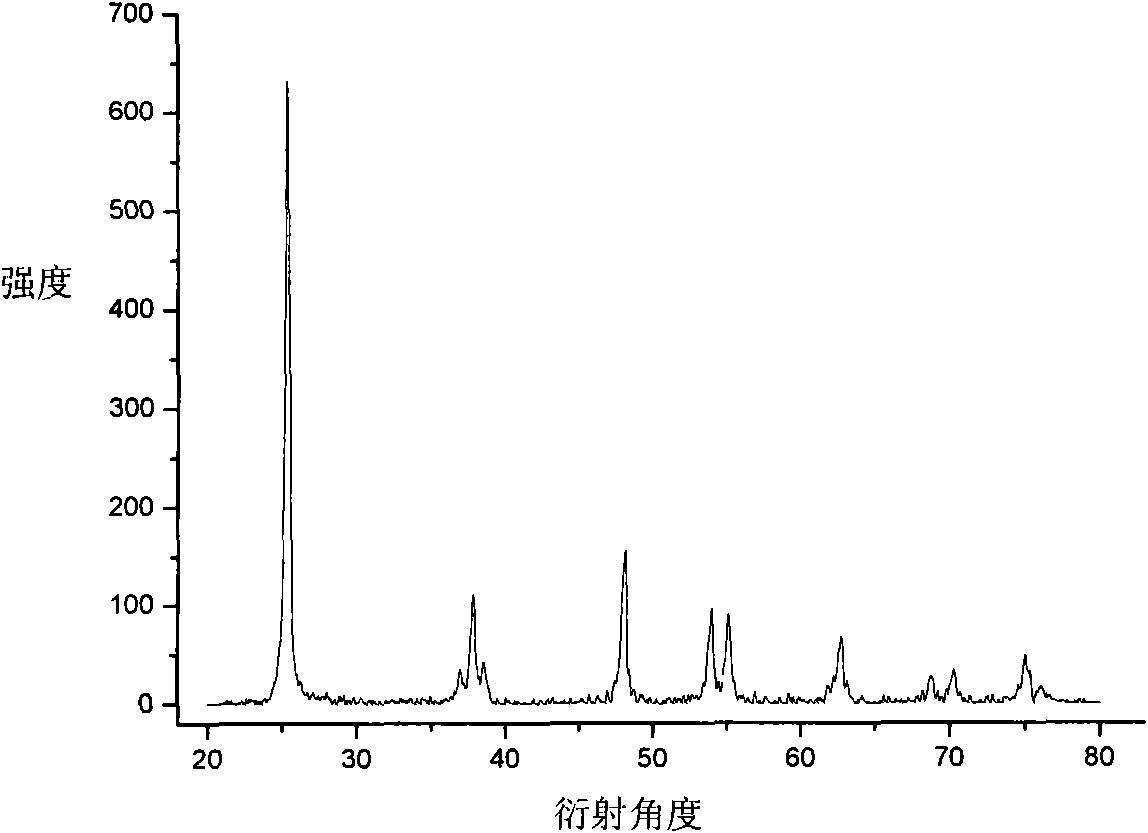
[0024] 3) After the precipitate is completely dried, it is heated to 450° C. and calcined for 1.5 h to obtain a nitrogen-doped nano-titanium dioxide photocatalyst. The color of the sample is light yellow, and it is recorded as 1# sample.
[0025] The structure of the 1# sample was detected by XRD. The sample was anatase nitrogen-doped nano-titanium dio...
Embodiment 2
[0027] 1) Configure 1mol / L titanium sulfate solution, add surfactant Tween under the condition of stirring, the mass of Tween added is 0.2% of the mass of titanium sulfate solution, after stirring evenly, add 5mol / L ammonia water to completely precipitate titanium sulfate , continue to stir for 2h, and leave to age for 10h;
[0028] 2) Wash the precipitate with deionized water until the total concentration of all anions in the washed water is lower than 0.1mol / L, and then vacuum-dry the precipitate in an environment with a pressure below 800Pa and a temperature below -5°C;
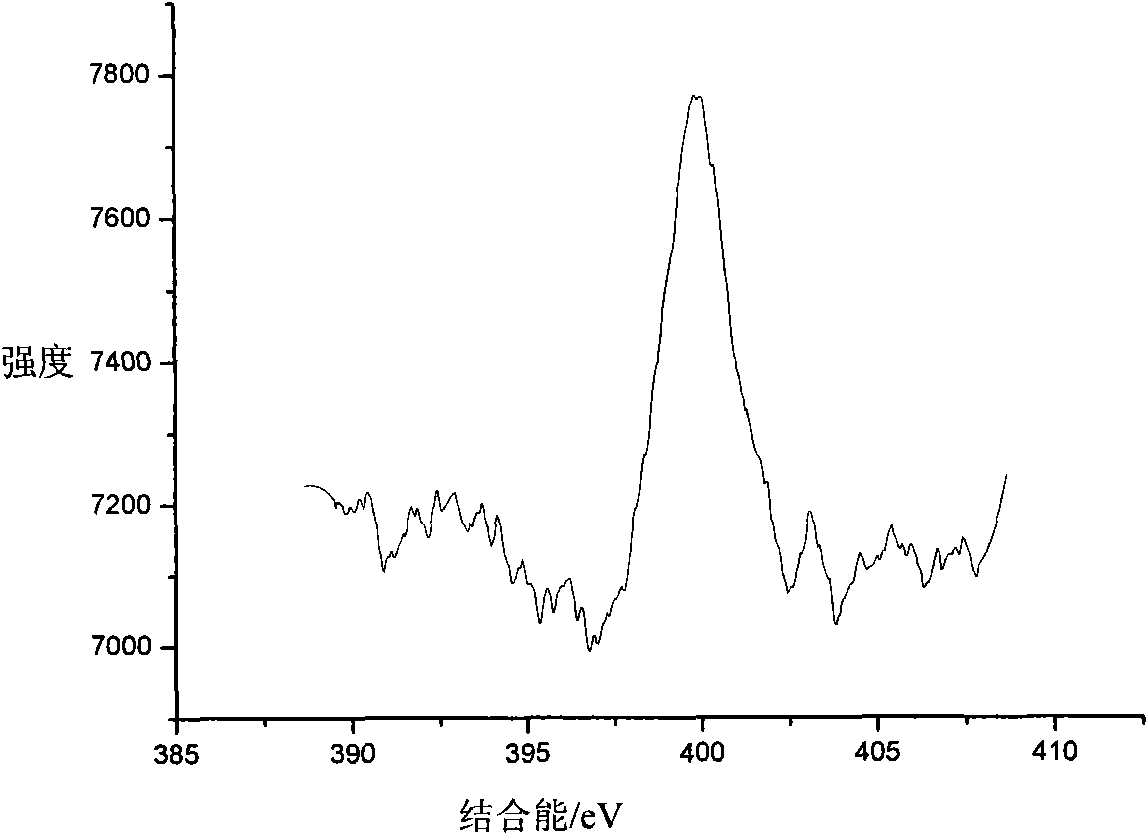
[0029] 3) After the precipitate is completely dried, it is heated to 800° C. and calcined for 0.5 h to obtain a nitrogen-doped titanium dioxide photocatalyst. The color of the sample is single yellow, which is recorded as 2# sample.
[0030] The structure of the 2# sample was detected by XPS, and the results showed that N doping entered TiO 2 within the lattice.
Embodiment 3
[0032] 1) Configure 10mol / L titanium tetrachloride solution, add surfactant polyethylene glycol under the condition of stirring, the quality of surfactant accounts for 0.3% of the total mass of titanium tetrachloride solution, add 2mol / L after stirring evenly ammonium bicarbonate aqueous solution to completely precipitate titanium tetrachloride, continue to stir for 2 hours, and leave to age for 24 hours;
[0033] 2) washing the precipitate with deionized water until the total concentration of all anions in the washed water is lower than 0.1mol / L, and then vacuum-drying the precipitate in an environment with a pressure below 200Pa and a temperature below -30°C;
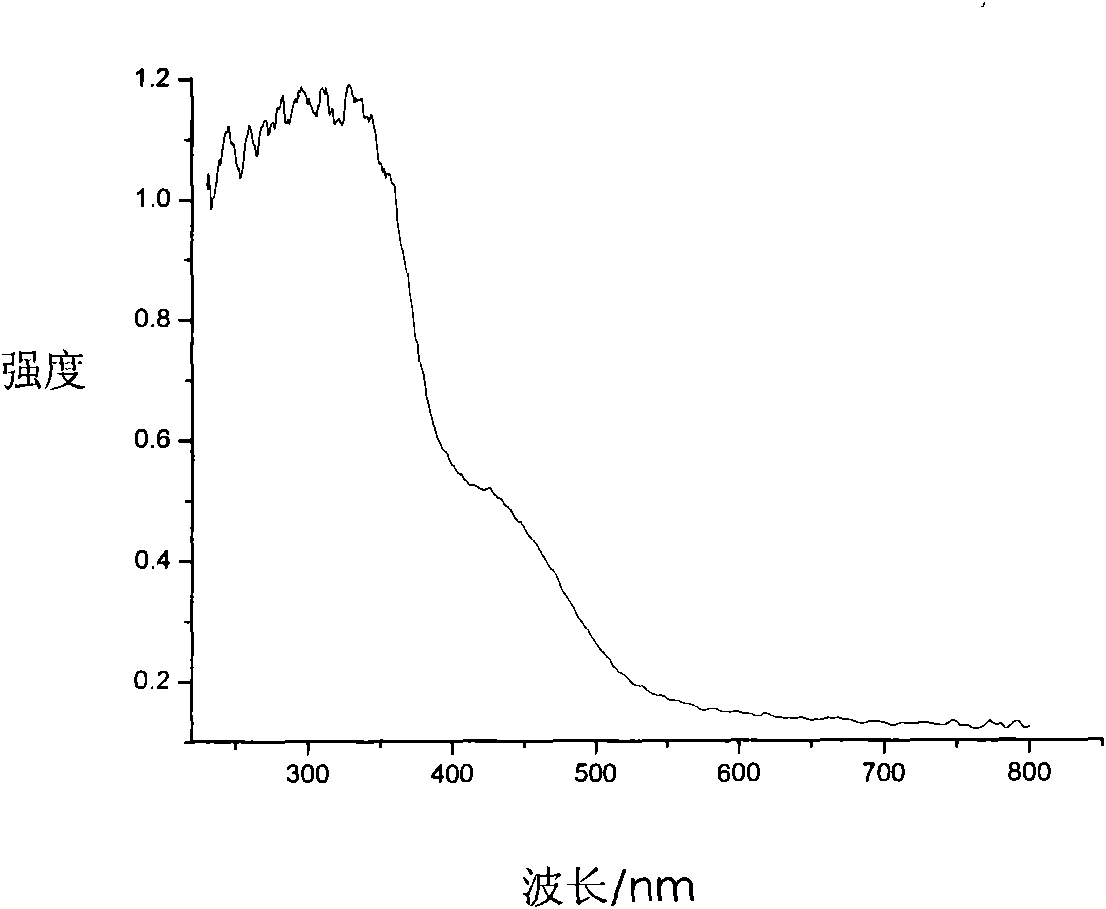
[0034] 3) After the precipitate is completely dried, it is heated to 500° C. and calcined for 1 hour to obtain a nitrogen-doped titanium dioxide photocatalyst. The color of the sample is bright yellow, which is recorded as 3# sample.
[0035] The spectral performance of the 3# sample is tested by ultraviolet-visible ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com