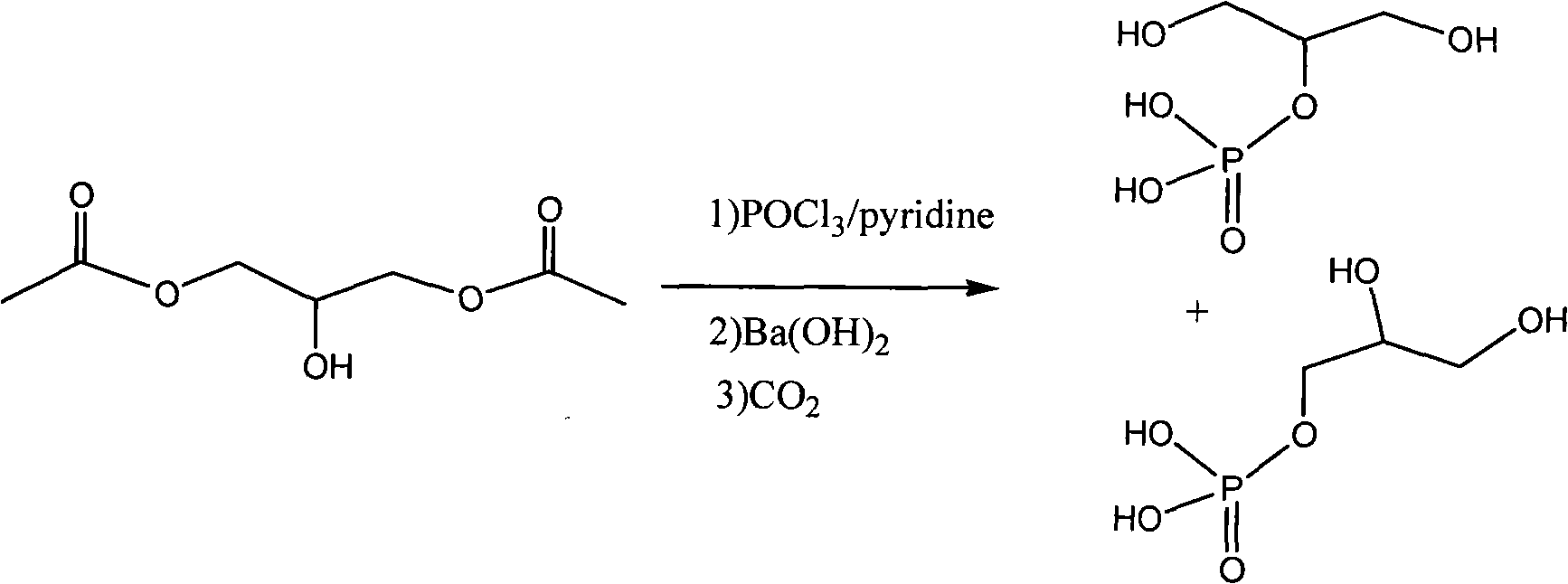
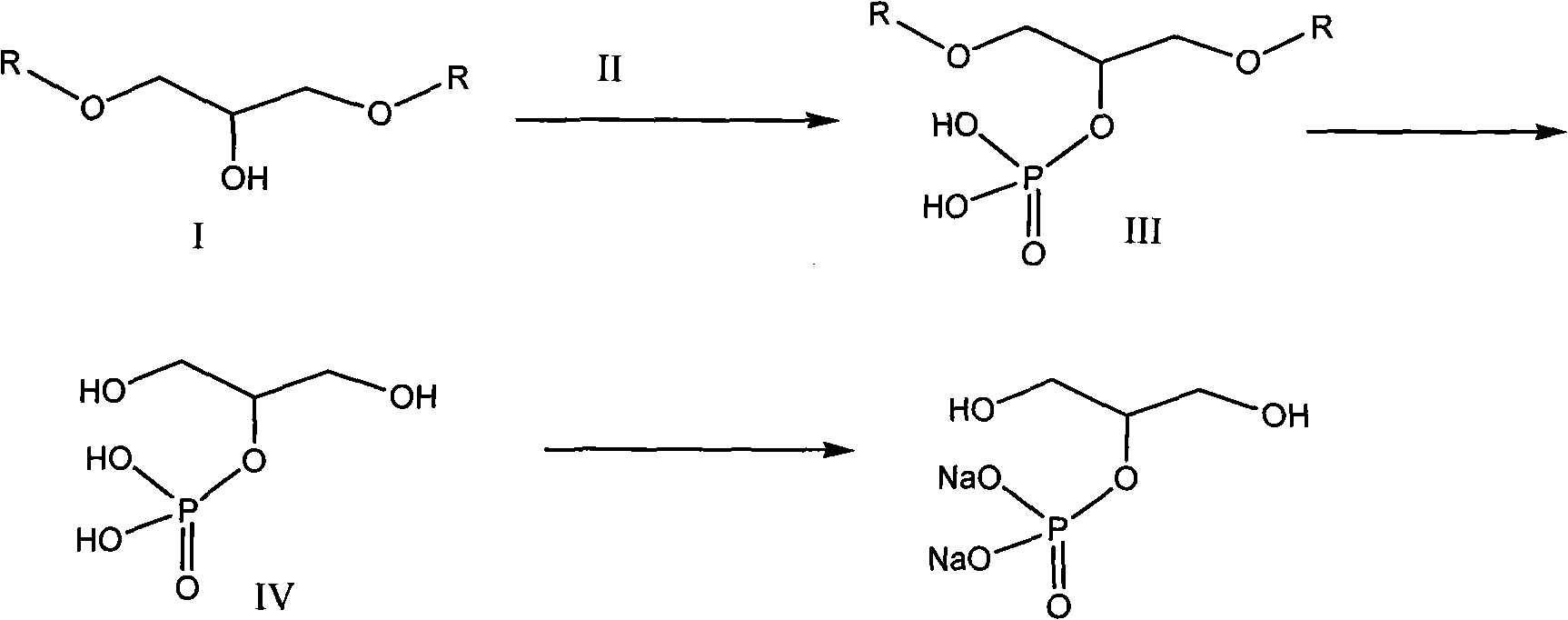
Method for synthesizing beta-sodium glycero-phosphate
A technology of sodium glycerophosphate and a synthesis method is applied in the field of synthesis of a phosphorus deficiency treatment agent or a nutritional medicine for phosphorus supplementation, and can solve the problems of only microgram level of separation and difficulty in industrial application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 1, the synthesis of 3-dibenzyloxy (or substituted benzyloxy)-2-propoxyphosphoric acid (III)
[0020] 1,3-dibenzyloxy (or substituted benzyloxy)-2-propanol (I) 54.4 g (0.20 mol) was dissolved in 200 ml of methylene chloride, 39.5 g of pyridine was added, cooled to -10°C 153.5 grams of phosphorus oxychloride was added dropwise under stirring, and after stirring for 1 hour at -10°C, it was allowed to rise naturally to room temperature and stirred for 3 hours. Stir for 1 hour, extract with 200 ml of ethyl acetate, wash the organic phase successively with 5% aqueous sodium bicarbonate solution, saturated brine, dry over anhydrous magnesium sulfate, evaporate the solvent to obtain 56.3 g of light yellow transparent liquid, yield: 80%. MS: 350.9 (ESI), HNMR: 7.20-7.25 (m, 10H), 5.42-5.45 (d, 4H), 3.61-3.64 (dd, 4H).
Embodiment 2
[0021] The synthesis of embodiment 2 β-sodium glycerophosphate
[0022] 1,3-Dibenzyloxy (or substituted benzyloxy)-2-propoxyphosphoric acid (III) 167 grams (0.47mol) was dissolved in 1200 ml of ethyl acetate, added to a 2-liter autoclave, and 8.3 grams of 10% palladium carbon, hydrogen replacement 3 times, hydrogen pressure added to 50 atmospheres, stirred at 50 ° C for 20 hours, added 500 ml of water, the aqueous layer was extracted 3 times with ethyl acetate (200 ml x 3), the aqueous layer Adjust Ph=9, remove most of the water by distillation under reduced pressure, freeze-dry the raffinate to obtain 125 grams of crude product of β-glycerophosphate sodium, recrystallize to obtain 115 grams of white crystals, yield 80%, content 99% (HPLC, external standard method ). Na: 21.75%, P: 14.70.%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com